Avelumab in the Treatment of Bladder Cancer: Current Insights and Future Prospects
- Article
- Article Info
- Author Info
Introduction
In recent years, the field of oncology has undergone a revolutionary transformation in therapeutic approaches, primarily driven by the introduction of immunotherapy. The advent of immune-based treatments has redefined the strategies for managing distinct types of cancers, including the intricate terrain of Bladder Cancer. As medical practitioners and researchers strive to optimize patient outcomes and improve their quality of life, the multifaceted landscape of Bladder Cancer management has unveiled distinctive challenges, prompting a quest for innovative treatment modalities.
Bladder Cancer, marked by its high prevalence and significant disease burden, has provided an ideal arena for the exploration of novel approaches. The integration of immunotherapeutic agents, particularly immune checkpoint inhibitors, such as Avelumab, has emerged as a promising avenue in the fight against this formidable malignancy. Avelumab, an immune checkpoint inhibitor targeting axis [1], has attracted considerable attention due to its potential to revolutionize the treatment landscape for Bladder Cancer.
The emergence of Avelumab as an effective therapeutic option has been underscored by pivotal clinical trials and real-world investigations that have illuminated its prospective impact. The landmark JAVELIN Bladder 100 trial showcased the substantial advantages of Avelumab as a first-line maintenance therapy for patients with advanced urothelial carcinoma (aUC). This endorsement as a maintenance therapy signifies a significant milestone in Bladder Cancer treatment, reshaping the standard of care for patients in advanced stages[2].
Immunotherapy’s role in managing bladder urothelial cancers is rapidly evolving, extending beyond its initial application in second-line therapy to encompass first-line metastatic disease and high-risk non-muscle-invasive bladder cancer refractory to BCG treatment. The investigation of Avelumab’s role in neoadjuvant and adjuvant settings for muscle-invasive bladder cancer further highlights its expanding scope.
However, the implementation of Avelumab and other immune checkpoint inhibitors comes with its share of challenges. The identification of suitable patients for these therapies, the management of potential adverse effects, and the optimization of treatment regimens remain areas of ongoing research. Moreover, as our comprehension of the intricate interplay between the tumor microenvironment, immune response, and therapeutic agents deepens, avenues for combination therapies and innovative biomarker-driven approaches are being explored.
This comprehensive review delves into the intricate landscape of Avelumab’s role in Bladder Cancer treatment. By amalgamating insights from pivotal trials like the JAVELIN Bladder 100 with perspectives from real-world studies, this review aims to provide a comprehensive grasp of Avelumab’s influence on patient outcomes. Furthermore, it delves into ongoing and anticipated clinical trials that are poised to shape the future of Bladder Cancer management, exploring neoadjuvant and adjuvant settings, patient selection criteria, and treatment strategies. The multifaceted nature of Avelumab’s impact is discussed, considering its potential in distinct stages of Bladder Cancer, from advanced to metastatic disease.
The prevalence and complexities of Bladder Cancer make it a significant global health concern, warranting continuous exploration of innovative therapeutic options. As this review navigates through the intricate facets of Avelumab’s role in Bladder Cancer treatment, it aims to provide a comprehensive guide for healthcare professionals and researchers, aiding them in understanding the evolving landscape of immunotherapy in this challenging domain.
Mechanism of Action of Avelumab in Bladder Cancer
Understanding the intricate workings of Avelumab’s mechanism of action is crucial to fully appreciate its impact on Bladder Cancer. Bladder Cancer, like many other malignancies, employs a range of tactics to evade the body’s immune surveillance. One of these strategies involves the upregulation of programmed death-ligand 1 (PD-L1), a key immune checkpoint protein, which effectively dampens the immune system’s ability to recognize and attack tumor cells[1].
Avelumab, a PD-L1 inhibitor, operates by disrupting this crucial immunosuppressive pathway. By binding to PD-L1 on tumor cells, Avelumab prevents its interaction with the programmed cell death protein 1 (PD-1) receptor on T cells. This pivotal interaction normally serves as a “brake” mechanism, restraining the immune system from mounting an excessive response against healthy cells. However, in the context of cancer, this interaction is often exploited by tumor cells to evade immune destruction.
By blocking the PD-L1/PD-1 interaction, Avelumab effectively releases the brake on the immune response. This enables activated T cells to recognize, infiltrate, and attack tumor cells with heightened precision and efficiency. The result is a reinvigorated immune response against the cancer, which can lead to tumor regression and improved patient outcomes.
Beyond its direct impact on the PD-L1/PD-1 axis, Avelumab’s effects extend to the tumor microenvironment itself. The presence of PD-L1 on tumor cells not only inhibits T cell activity, but it also plays a role in shaping the local immune landscape within the tumor. By disrupting this interaction, Avelumab can create an environment that is less conducive to immune evasion. This modulation of the tumor microenvironment contributes to the development of a more robust and sustained immune response against the cancer.
In summary, Avelumab’s mechanism of action in Bladder Cancer revolves around its ability to restore the immune system’s recognition and targeting of tumor cells. By inhibiting the PD-L1/PD-1 interaction, Avelumab reactivates the immune response, while also shaping a tumor microenvironment that is less permissive to immune evasion. This comprehensive approach makes Avelumab a promising and potent tool in the fight against Bladder Cancer, potentially offering improved outcomes and new avenues for patient care.
PD-L1 and its Role in Immune Response:
The intricate interplay between tumor cells and the immune system plays a pivotal role in the development and progression of bladder cancer. One key strategy employed by cancer cells is the elevated expression of the programmed death-ligand 1 (PD-L1) protein, which acts as a shield, allowing cancer cells to evade the immune response. PD-L1 binds to the programmed death-1 receptor (PD-1) on activated T cells, sending inhibitory signals that suppress T cell activity, dampening the body’s antitumor immune response. While the immune system requires a delicate balance of stimulation and inhibition signals to protect the body from external threats and prevent self-destructive attacks, cancer cells exploit these inhibitory mechanisms to evade immune recognition and thrive. Immunotherapy has emerged as a promising approach to reinvigorate the immune system’s response against cancer. Specifically, targeting immune checkpoints like the programmed cell death pathway (PD-1/PD-L1) has gained significant attention. Blocking the interaction between PD-1 and PD-L1 can restore the immune response mechanisms, enhancing the body’s ability to fight tumors. Monoclonal antibodies that inhibit PD-1 or PD-L1 have demonstrated clinical efficacy. Ongoing research aims to unravel the complexities of the PD-1/PD-L1 pathway, identifying new factors that influence its activity and understanding the pathway’s variability within the tumor microenvironment. These findings hold the potential to optimize anticancer strategies by targeting the PD-1/PD-L1 signaling pathway effectively in clinical practice.[3].
PD-L1 Blockade with Avelumab: Enhancing Antitumor Immune Response
Avelumab, an immune checkpoint inhibitor, aims to disrupt this immune evasion mechanism by blocking the interaction between PD-L1 and PD-1. By binding to PD-L1 on tumor cells, Avelumab prevents the inhibitory signal directed at T cells, triggering a more robust and effective immune response against tumor cells. This process leads to the activation of cytotoxic T cells, which are capable of specifically recognizing and eliminating cancer cells[3, 4].
Impact on Tumor Microenvironment and Host Response
PD-L1 blockade with Avelumab not only has a direct impact on tumor cells but also modulates the tumor microenvironment and host response. The tumor microenvironment in Bladder Cancer is often characterized by the presence of immunosuppressive cells and factors inhibiting immune response. Avelumab alters this balance by enhancing T cell activity and reducing the presence of immunosuppressive cells. Additionally, it has been observed that PD-L1 blockade with Avelumab increases the infiltration of immune cells into the tumor, suggesting a more active immune response in the tumor microenvironment[4, 5]. [Figure 1].
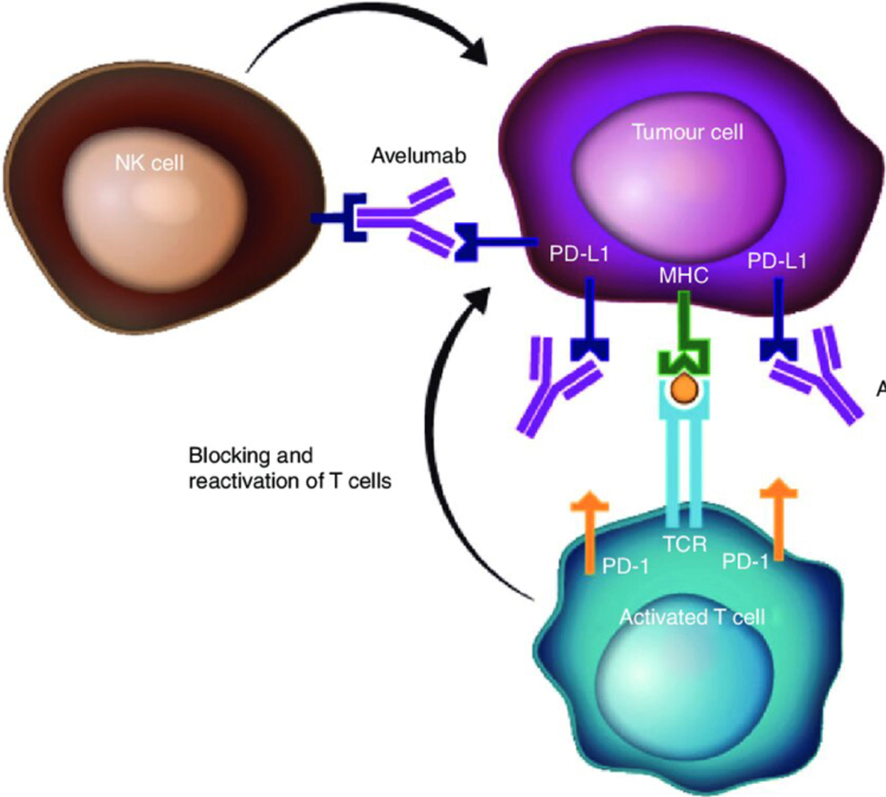
Figure 1: Avelumab’s dual mechanism of action. Avelumab is a human IgG1 monoclonal antibody that specifically binds to PD-L1, preventing the interaction between PD-L1 and the inhibitory T-cell receptor, PD-1. PD-L1 blockade removes the suppression of T-cell activity, resulting in T-cell-mediated, adaptive antitumour immune responses. In addition, avelumab has a wild-type IgG1 Fc region that may enable NK cell-mediated ADCC. (“Avelumab: clinical trial innovation and collaboration to advance anti …”) Avelumab therefore has the potential to utilise both adaptive and innate immune mechanisms to destroy cancer cells. ADCC, antibody-dependent cell-mediated cytotoxicity; Fc, crystallisable fragment; IgG1, immunoglobulin G1; MHC, major histocompatibility complex; NK, natural killer; PD-1, programmed death-1; PD-L1, programmed death ligand-1; TCR, T-cell receptor.
Clinical Outcomes and Efficacy of Avelumab in Bladder Cancer
The clinical evidence supports the positive impact of Avelumab on Bladder Cancer. The JAVELIN Bladder 100 clinical trial demonstrated that the combination of Avelumab with chemotherapy resulted in a significant improvement in overall survival compared to chemotherapy alone. The median overall survival was 21.4 months in the combination group compared to 14.3 months in the chemotherapy-alone group (HR 0.69, 95% CI: 0.56-0.86, p < 0.001). These findings indicate a new dimension in Bladder Cancer treatment, offering a promising therapeutic option for patients seeking more effective and less invasive alternatives[6].
“JAVELIN Bladder 100” Study: This study focused on administering Avelumab after platinum chemotherapy in patients with advanced urothelial carcinoma (aUC). It found that adding Avelumab to supportive care significantly improved overall survival (OS) compared to supportive care alone. This improvement persisted even with a higher number of subsequent treatments in the control group, suggesting early initiation of Avelumab is crucial. Additionally, patients with positive PD-L1 expression showed greater benefits, but the benefit also remained in the overall population. These findings align with earlier studies showcasing the efficacy of immune checkpoint inhibitors (ICIs) in aUC treatment[6, 7].
“JAVELIN Bladder 100 Clinical Subgroup Analyses”: This study performed subgroup analyses to evaluate Avelumab’s efficacy in various clinical contexts. Results indicated that Avelumab’s benefit remained consistent across several subgroups, including those with different chemotherapy regimens and PD-L1 status. This supports the idea that Avelumab is an effective option for a wide range of aUC patients, regardless of their clinical characteristics[8].
“Maintenance Avelumab After PBC in Real-World aUC Patients”: The results of this real-world study align with clinical trial conclusions and other real-world studies like READY and AVENANCE. Data demonstrated that overall survival and progression-free survival were in line with previous findings. This underscores the clinical utility of Avelumab in real-world settings and aligns with findings from more controlled clinical trials[9, 10].
“Role of Maintenance Therapy in Urothelial Cancer”: This article highlights the importance of immune checkpoint inhibitor (ICI) maintenance therapy in metastatic urothelial carcinoma. The results of JAVELIN Bladder 100 set a new standard by demonstrating that Avelumab as post-platinum chemotherapy maintenance therapy extends survival. This is particularly relevant as before these results, there was no established maintenance strategy after first-line chemotherapy. Additionally, the article emphasizes the need to optimize treatment as the disease progresses, including exploring therapeutic combinations[10, 11].
In summary, the presented studies provide robust evidence that Avelumab as maintenance therapy after platinum chemotherapy improves survival in patients with advanced urothelial carcinoma. The results mutually support each other in terms of efficacy and safety, both in clinical trials and real-world situations. Furthermore, it underscores the importance of considering multiple clinical factors when deciding on treatment and the need to continue researching to optimize management strategies for aUC.
Pioneering Clinical Studies: Evidence of Efficacy
The assessment of Avelumab’s efficacy in the treatment of Bladder Cancer has been based on rigorous clinical trials that have yielded solid evidence of its positive impact on patients’ clinical outcomes. Among these pioneering studies, the JAVELIN Bladder 100 trial stands out, which evaluated the efficacy of Avelumab in patients with advanced metastatic Bladder Cancer who had responded or stabilized after first-line chemotherapy. This study demonstrated a significant benefit in overall survival with the addition of Avelumab compared to chemotherapy alone. The median overall survival in the Avelumab group was 21.4 months compared to 14.3 months in the chemotherapy-alone group, resulting in a 31% reduction in the risk of death (HR 0.69, 95% CI: 0.56-0.86, p < 0.001)[7, 8, 12-14].
Objective Responses and Survival in Bladder Cancer Patients
In addition to overall survival, significant objective responses have been observed in patients treated with Avelumab. In the JAVELIN Bladder 100 trial, the objective response rate in the Avelumab group was 47.4% compared to 31.4% in the chemotherapy-alone group. The responses were durable and were maintained over prolonged periods of time, highlighting the sustainability of therapeutic benefits. Furthermore, Avelumab demonstrated an improvement in progression-free survival rate compared to chemotherapy alone (HR 0.71, 95% CI: 0.59-0.86, p < 0.001), underscoring Avelumab’s ability to control disease progression and prolong response duration[7, 8, 15].
Comparison with Conventional Therapeutic Options: A New Dimension
The incorporation of Avelumab into the treatment of Bladder Cancer has opened a new dimension compared to conventional therapeutic options. Traditionally, treatment choices after first-line chemotherapy in patients with metastatic Bladder Cancer have been limited. The introduction of Avelumab has shifted this landscape by offering an effective and well-tolerated alternative. Compared to chemotherapy alone, Avelumab not only improves overall survival but also provides durable objective responses and a significant reduction in the risk of disease progression. This enhancement in clinical outcomes positions Avelumab as an essential therapeutic option for this patient population [Table 1].
Table 1: Efficacy results from the mentioned studies on Avelumab in Advanced Urothelial Carcinoma
| Study | Efficacy Results |
| Javelin Bladder 100-patient-Reported Outcomes | Avelumab+BSC group had no significant reduction in quality of life vs BSC alone |
| Time to deterioration of symptoms was comparable between groups. | |
| Some specific symptom differences were not clinically significant | |
| Avelumab group reported higher side effect frequency but not impacting overall quality of life | |
| Javelin Bladder 100-updated findings After >2 years of follow up | Avelumab+BSC showed prolonged overall survival (mOs 21.4vs.14.3months) over BSC alone |
| Safety profile remained consistent with earlier analysis | |
| Safety profile remained consistent with earlier analysis | |
| Javelin Bladder 100-comprehensive clinical subgroup analysis | Hazard ratio for os with Avelumab+BSC consistently <1.0 across all examined subgroups |
| Efficacy benefits were observed across diverse patient characteristics | |
| The role of switch maintenance therapy in urothelial cancers | Maintenance Avelumab prolongs OS and PFS in mUc post platinum based chemotheraphy |
| Trials explore combination of avelumab with targeted therapies and novel agents | |
| Response and outcomes of maintenance avelumab after platinum-based chemotherapy (PBC) in a Auc: “Real World” | Median progression free survival (Mpfs) with avelumab maintenance was 9.6 months |
| Experience | Estimated one-year overall survival (OS) rate was 72.5% |
| Positive prognostic factors include CR/PR to 1L (PBC) and ECOG PS of 0. | |
| ORR to avelumab maintenance was 28.7% with CR and PR observed. |
Challenges and Considerations in the Clinical Use of Avelumab in Bladder Cancer
Despite encouraging results, there are challenges and considerations in the clinical use of Avelumab in Bladder Cancer. The selection of appropriate patients is crucial to maximize therapeutic benefits. Although PD-L1 expression has been linked to Avelumab response, its predictive value is still being solidified. Exploring additional biomarkers such as tumor mutational burden and the presence of infiltrating T cells is essential to identify patients who will optimally respond to the therapy. Furthermore, managing autoimmune adverse effects like dermatitis and colitis requires early intervention and multidisciplinary management to ensure the safety and tolerability of the therapy[14]. [Table 2].
Table 2: “Key Messages and Their Implications in Clinical Practice”
| Key Messages | Implications in Clinical Practice |
| Avelumab has revolutionized the treatment of advanced metastatic Bladder Cancer. | Healthcare professionals should consider Avelumab as an important therapeutic option for patients with advanced Bladder Cancer, particularly in refractory or progressive cases after conventional therapies. |
| Immunotherapy with Avelumab improves overall survival and objective responses. | Physicians should discuss with patients the potential benefits of immunotherapy with Avelumab in terms of survival and quality of life. |
| Avelumab effectively combines with chemotherapy and other immune checkpoint inhibitors. | The combination of Avelumab with other therapies opens new treatment perspectives. Clinicians should stay updated on approved combinations and investigational options to optimize therapeutic strategies. |
| Predictive biomarkers like PD-L1 expression guide patient selection for immunotherapy. | Identifying predictive biomarkers is crucial to personalize therapy and enhance response. Physicians should consider evaluating PD-L1 and other biomarkers when selecting patients for Avelumab. |
| Managing autoimmune adverse effects requires deep understanding and multidisciplinary management. | Medical teams should be trained in managing autoimmune adverse effects and be prepared to address the5m effectively. Communication with patients is essential to ensure safe and tolerable therapy. |
| Immunotherapy will continue to evolve and challenge medicine in identifying new targets. | Healthcare professionals should stay current with developments in immunotherapy and be willing to adapt their practices based on new therapies and emerging therapeutic approaches. |
Patient Selection: Identifying Ideal Candidates
The successful incorporation of Avelumab into the clinical practice of Bladder Cancer faces the fundamental challenge of identifying suitable patients for this therapy. Although Avelumab has demonstrated efficacy in clinical trials, not all patients achieve the same benefits. Selecting ideal candidates is essential to optimize clinical outcomes and avoid unnecessary treatments. PD-L1 expression on tumor cells has been a traditionally used biomarker to predict response to immunotherapy. However, the predictive value of PD-L1 in Bladder Cancer is still inconclusive, as Avelumab response can be observed in tumors with both high and low PD-L1 expression. Therefore, identifying ideal candidates should be based on multiple clinical and biological factors to ensure the appropriate choice of therapy.
In the context of advanced urothelial carcinoma (aUC), the phase 3 JAVELIN Bladder 100 trial investigated the effectiveness of avelumab first-line (1L) maintenance therapy alongside best supportive care (BSC). The trial demonstrated significant improvements in overall survival (OS) and progression-free survival (PFS) compared to BSC alone in patients who had not progressed following 1L platinum-based chemotherapy[15].
This study delves into comprehensive clinical subgroup analyses from the trial. The results, although exploratory, showcased consistent trends in OS and PFS across various clinical subgroups, reaffirming the efficacy of avelumab 1L maintenance. Notably, patients with complete response (CR) after 1L chemotherapy displayed immature data, indicating the potential benefit of avelumab in maintaining durable responses. The study also shed light on the challenges of predicting disease control, emphasizing the importance of optimizing 1L treatments, especially given the limited use of second-line therapies in real-world scenarios. These findings underscore the relevance of avelumab 1L maintenance as a standard of care for diverse patients with advanced UC who have not progressed after 1L platinum-based chemotherapy, regardless of specific clinical characteristics.
The analysis of the JAVELIN Bladder 100 trial’s subgroups provides compelling evidence in support of avelumab 1L maintenance therapy for advanced urothelial carcinoma. Despite the complexity of diverse patient profiles, the consistent positive outcomes in OS and PFS underline the significance of this treatment approach. These findings not only validate the use of avelumab across a range of clinical subgroups but also emphasize the critical need for such therapies, especially in patients without reliable predictive biomarkers for disease control. Avelumab 1L maintenance stands as a pivotal standard of care for patients with advanced UC, further highlighting the imperative nature of optimizing initial treatments in the absence of robust predictors for long-term benefits.[8, 16]
Predictive Biomarkers: Implications for Personalized Therapy
The search for more precise predictive biomarkers is a critical component in the clinical use of Avelumab in Bladder Cancer. Identifying reliable biomarkers will allow for a more accurate patient selection for therapy, thereby avoiding exposure to ineffective treatments and reducing associated costs and adverse effects. In addition to PD-L1, tumor mutational burden and the presence of infiltrating T cells have also been proposed as potential biomarkers. Future studies should investigate the clinical utility of these biomarkers and their ability to predict Avelumab response and guide personalized therapy.
A study explores the complex landscape of biomarkers for immune checkpoint inhibitors (ICIs) in advanced bladder cancer (BCa). Historically, PD-L1 expression has been studied extensively, but its predictive role has varied across trials due to inconsistent detection methods. Notably, in Study 1108, ORR was significantly higher in patients with PD-L1-positive tumor or immune cells when treated with durvalumab. However, other ICIs like nivolumab and pembrolizumab showed mixed results regarding PD-L1 positivity and response rates. Beyond PD-L1, VISTA expression in myeloid cells, granulocytes, and T-cells has been suggested as a potential immunological biomarker for BCa. Additionally, Tumor Mutational Burden (TMB) emerged as a reliable predictor for ICI response, with higher TMB correlating with better responses and longer overall survival. Molecular subtyping and gene-expression profiling further delineated BCa into diverse subtypes, indicating the need for a multifaceted approach in biomarker exploration. These findings emphasize the necessity of understanding the intricate interplay between biomarkers and ICIs in BCa, providing valuable insights for personalized immunotherapy strategies[17].
Management of Adverse Effects: Optimizing Safety and Tolerance
The management of autoimmune adverse effects associated with Avelumab is another critical challenge in its clinical use. While Avelumab has a manageable safety profile, autoimmune adverse effects such as dermatitis, colitis, and organ-specific autoimmune reactions can limit its tolerability and impact patients’ quality of life. Early recognition and appropriate management of these adverse effects are essential to prevent serious complications. Multidisciplinary collaboration among oncologists, immunologists, and other specialists is crucial to optimize the safety and tolerability of the therapy, allowing patients to receive the therapeutic benefits of Avelumab while minimizing adverse effects[2, 16, 18, 19].
Combination of Treatments and Future Directions with Avelumab in Bladder Cancer
Therapeutic Synergy: Exploration of Promising Combinations
The combination of Avelumab with other therapeutic modalities presents an exciting strategy to enhance the immune response and improve clinical outcomes in patients with Bladder Cancer. One of the most promising areas of research is the combination of Avelumab with chemotherapy. In studies like the JAVELIN Bladder 100 trial, the combination of Avelumab with chemotherapy demonstrated a significant improvement in overall survival compared to chemotherapy alone, setting a new standard in second-line treatment. This therapeutic synergy suggests that combining Avelumab with conventional therapies can enhance antitumor efficacy and improve clinical outcomes in Bladder Cancer[20-22].
Innovations in Immune Therapy: Future Developments
The horizon of immune therapy in Bladder Cancer is filled with promise. In addition to combinations with conventional therapies, innovations in immunotherapy are being explored that could further revolutionize the treatment of this disease. One such innovation is the combination of Avelumab with other immune checkpoint inhibitors like ipilimumab, which has shown promise in clinical studies. This combination can maximize T cell activation and trigger more potent and durable immune responses. Furthermore, adoptive cell therapy and personalized vaccines are emerging areas of research that could offer new strategies to enhance the immune response in Bladder Cancer[17, 23].
Ongoing Contributions to the Fight Against Cancer: Promising Perspectives
The prospects for the use of Avelumab in Bladder Cancer are encouraging. As we continue to learn about mechanisms of action and immune interactions, we are in a privileged position to develop more precise and effective therapeutic approaches. The combination of treatments and innovations in immune therapy are transforming how we approach this disease. As more evidence accumulates and innovative studies are conducted, we are likely to see further expansion in therapeutic options and an even greater impact on the clinical outcomes of patients with Bladder Cancer[24].
Discussion
Impact of Avelumab in Bladder Cancer: Clinical and Practical Considerations
The incorporation of Avelumab in the treatment of Bladder Cancer has transformed the way we approach this disease. Positive clinical outcomes observed in pioneering clinical trials, such as the JAVELIN Bladder 100 trial, have set a new standard in second-line treatment. The improvement in overall survival and sustained objective responses demonstrate that Avelumab is a valuable therapeutic tool for patients with advanced metastatic Bladder Cancer. However, challenges such as selecting appropriate patients for this therapy, identifying predictive biomarkers, and managing autoimmune adverse effects remain crucial. Informed clinical decision-making and multidisciplinary collaboration are essential to optimize the use of Avelumab and ensure the best outcomes for patients[2].
A study conducted a comprehensive medical chart review of patients with advanced urothelial carcinoma (UC) who had not progressed after first-line platinum-based chemotherapy, comparing their demographics, clinical features, and treatment histories with those enrolled in the phase 3 JAVELIN Bladder 100 (JB-100) trial. The analysis demonstrated remarkable similarities in patient characteristics between the real-world MCR cohort and the JB-100 trial participants. Specifically, the gender distribution, number of chemotherapy cycles, and Eastern Cooperative Oncology Group performance status were consistent. Furthermore, approximately 75% of MCR patients achieved a complete or partial response with platinum-based chemotherapy, reinforcing the alignment with the JB-100 trial. These findings suggest that the JB-100 trial population effectively represents patients eligible for avelumab first-line maintenance therapy in real-world settings. Future research endeavors should focus on validating these observations, exploring the consistency of real-world outcomes with the trial’s findings[10].
Combined Therapies and Biomarkers: Perspectives for Treatment Optimization
The combination of Avelumab with other therapeutic modalities and the identification of predictive biomarkers represent exciting areas of research in Bladder Cancer. The therapeutic synergy observed in combination with chemotherapy and other immune checkpoint inhibitors opens the door to more potent and effective therapeutic approaches. Furthermore, the search for more precise biomarkers will enable a more accurate patient selection and personalized therapy. Tumor mutational burden, PD-L1 expression, and the presence of infiltrating T cells are promising candidates to guide therapy selection. Ongoing research in these areas is essential to optimize clinical outcomes and tailor treatment for each patient.
Considerations in Administration and Access to Avelumab Therapy
Despite therapeutic advancements, considerations in the administration and access to Avelumab are essential to ensure that patients benefit from this therapy. Educating healthcare professionals and providing training in adverse effect management are critical to optimizing safety and tolerability. Additionally, access to Avelumab and other immune therapies remains a challenge in many regions. Addressing financial and logistical barriers is fundamental to ensuring that all eligible patients can receive innovative treatments. Collaboration among governments, pharmaceutical companies, and healthcare organizations is essential to overcome these obstacles and improve access to Avelumab therapy.
Future Perspectives
Development of Novel Predictive Biomarkers
The future of Bladder Cancer treatment with Avelumab focuses on identifying more precise predictive biomarkers. As our understanding of immune response mechanisms evolves, opportunities arise to discover biomarkers that can more reliably predict patients’ response to therapy. The expression of PD-L1 and other emerging biomarkers, such as tumor mutational burden and infiltrating T cell presence, are being investigated in clinical and preclinical studies to determine their value in patient selection and personalized treatment guidance. Developing more precise biomarkers will allow for more effective patient stratification and optimization of therapeutic outcomes[17, 25, 26].
Innovative Combined Therapies: Expanding Therapeutic Options
The combination of Avelumab with other therapies presents an exciting avenue to expand therapeutic options in Bladder Cancer. In addition to combinations with chemotherapy and other immune checkpoint inhibitors, combined therapies with targeted agents and adoptive cellular treatments are on the horizon. Strategically combining therapies has the potential to further enhance immune response, address tumor heterogeneity, and overcome therapy resistance. These innovative combinations are being explored in clinical trials and could lead to significant advancements in Bladder Cancer treatment in the future[23, 27].
Continued Role of Immunotherapy in the Evolution of Bladder Cancer Treatment
Immunotherapy has marked a milestone in the evolution of Bladder Cancer treatment and is expected to continue playing a pivotal role in the future. Ongoing research into the interaction between the immune system and tumor cells will provide new opportunities to design more effective and precise therapeutic approaches. Adapting immunotherapy as new mechanisms and therapeutic targets are discovered will allow for ongoing evolution in Bladder Cancer management. As more targeted therapies are developed and new combinations are identified, immunotherapy will continue to contribute to improved clinical outcomes and the transformation of patient care.
A New study delves into the effectiveness and toxicity of checkpoint inhibitors in urothelial carcinoma through analysis of 21 prospective trials. It reveals varying efficacy and toxicity profiles of CPIs in metastatic UC, muscle-invasive UC, and non-muscle-invasive bladder cancer. Switch maintenance with avelumab post-chemotherapy emerges as the current standard for first-line metastatic UC. Pembrolizumab and atezolizumab are approved in the United States and Europe, respectively, for platinum-ineligible patients. Adjuvant nivolumab has demonstrated improved disease-free survival in high-risk muscle-invasive UC. The study underscores the need for further investigations into CPI combinations and patient selection to optimize UC treatment.
This comprehensive analysis highlights the diverse applications of checkpoint inhibitors in urothelial carcinoma. Current standards involve CPIs as post-chemotherapy maintenance and adjuvant treatments in specific high-risk cases. Ongoing trials exploring CPI combinations across various UC stages are expected to refine treatment approaches. The validation of potential biomarkers holds promise for personalized therapies, guiding future patient selection and resistance management in UC treatment strategies[28].
Conclusions
Transformation of the Therapeutic Landscape: Avelumab as an Innovative Agent
The introduction of Avelumab in Bladder Cancer treatment has represented a milestone in the history of oncology, marking the beginning of a new therapeutic era. The demonstrated efficacy in pioneering clinical trials and regulatory approval in multiple regions confirm Avelumab as an innovative agent in the therapeutic arsenal against advanced metastatic Bladder Cancer. Improved survival outcomes and sustained objective responses have redefined treatment expectations and provide hope for patients facing this devastating disease.
Challenges and Opportunities in the Era of Immunotherapy
A New trial-level analysis focused on assessing the relationship between surrogate endpoints (SEs), such as progression-free survival (PFS) and objective response rate (ORR), and overall survival (OS) in metastatic urothelial cancer (MUC). Among the evaluated SEs, PFS exhibited a moderate level of surrogacy for OS, with a hazard ratio for PFS (HRPFS) of ≤0.41 providing 95% confidence of OS improvement. However, ORR was found to be a poor surrogate for OS, regardless of the analysis method used. Factors like trial duration, crossover between control and treatment arms, and the line of therapeutic treatment influenced the correlation between SEs and OS. Despite the challenges associated with SEs, this study emphasizes the importance of rigorous validation and consideration of specific parameters, such as proportional hazard regression, when utilizing SEs in clinical trials, especially in the context of metastatic urothelial cancer[14, 27].
Despite the exciting advancements in Avelumab-based immunotherapy, this era also presents unique challenges and opportunities. The identification of more precise predictive biomarkers is crucial for personalizing therapy and maximizing clinical outcomes. Managing autoimmune adverse effects and selecting suitable patients for therapy require a deep understanding of the mechanisms of action and multidisciplinary collaboration. As new therapeutic combinations and innovative approaches emerge, optimizing therapy and proper patient selection will remain areas of ongoing research and development.
A Promising Future for Bladder Cancer Patients: Sustained Contributions of Avelumab
The future for Bladder Cancer patients appears promising due to the sustained contributions of Avelumab and immunotherapy in general. Ongoing research in identifying new biomarkers, exploring combined therapies, and adapting immunotherapy as new therapeutic targets are discovered ensures further advancements in treatment and patient care. Avelumab has paved the way for a new era in the fight against Bladder Cancer, and its impact will continue to resonate within the medical community and the lives of patients in the years to come[27].
Combination of Treatments and Future Directions with Avelumab in Bladder Cancer
The combination of Avelumab with other therapeutic modalities is an active line of research aiming to maximize immune response and improve clinical outcomes. Combined therapy with chemotherapy and radiation has shown promising synergy. Additionally, combinations with other immune checkpoint inhibitors, such as ipilimumab, have demonstrated encouraging response rates in clinical trials. These investigations raise exciting prospects for further enhancing therapeutic efficacy and patients’ quality of life[24].
References
- Ribas A, Wolchok JD (2018) Cancer immunotherapy using checkpoint blockade. Science 2018. 359: 1350-1355.
- Grivas P, Sumantha P, Devgan P, Joaquim B, Cora n et al, (2021) Avelumab first-line maintenance in locally advanced or metastatic urothelial carcinoma: Applying clinical trial findings to clinical practice. Cancer Treat Rev 97: 102187.
- Yanyan Han, Dandan Liu, Lianhong Li, (2016) The Role of PD-1/PD-L1 Signaling Pathway in Antitumor Immune Response. Klin Onkol 29: 72-77.
- Rouanne, M, Dean F, Thomas P, Padmanee S, Andrew N, et al, (2020) Rationale and Outcomes for Neoadjuvant Immunotherapy in Urothelial Carcinoma of the Bladder. Eur Urol Oncol 3: 728-738.
- Kumagai S, Yosuke T, Kamadia (2020) The PD-1 expression balance between effector and regulatory T cells predicts the clinical efficacy of PD-1 blockade therapies. Nat Immunol 21: 1346-1358.
- Powles T, Park H, Caserta C, Anders U, Costa N, (2020) et al., Avelumab Maintenance Therapy for Advanced or Metastatic Urothelial Carcinoma. N Engl J Med 383: 1218-1230.
- Powles T, Park H, Caserta C, Anders U, Costa N (2023) Avelumab First-Line Maintenance for Advanced Urothelial Carcinoma: Results From the JAVELIN Bladder 100 Trial After >/=2 Years of Follow-Up. J Clin Oncol 41: 3486-3492.
- Grivas P, Ullen A, Shilpa G, Bo H, Powels T (2023) et al., Avelumab First-line Maintenance Therapy for Advanced Urothelial Carcinoma: Comprehensive Clinical Subgroup Analyses from the JAVELIN Bladder 100 Phase 3 Trial. Eur Urol 84: 95-108.
- Bakaloudi DR, Talukder R, Grivas P, Y Yu, Stewart R et al, (2023) Response and Outcomes of Maintenance Avelumab After Platinum-Based Chemotherapy (PBC) in Patients With Advanced Urothelial Carcinoma (aUC): “Real World” Experience. Clin Genitourin Cancer 21: 584-593.
- Bellmunt J, Chang J, Kirkar P, Mayanak A, Sean D et al. (2023) Evaluating Real-World Characteristics of Patients with Advanced Urothelial Carcinoma Eligible for Avelumab Maintenance Therapy: A Multicountry Retrospective Medical Chart Review. Clin Genitourin Cancer 21: 459-466.
- Tan X (2023) The role of maintenance therapy in the treatment of advanced urothelial cancer: a comprehensive systematic review and network meta-analysis. J Chemother 35: 505-513.
- Powles T, Claudia C, Sridhar S, Demey W, et al, (2022) Plain language summary of results from the JAVELIN Bladder 100 study: avelumab maintenance treatment for advanced urothelial cancer. Future Oncol 18: 2361-2371.
- Heery CR, Madan A, Marte L, Anja V, Gulley L, et al, (2017) Avelumab for metastatic or locally advanced previously treated solid tumours (JAVELIN Solid Tumor): a phase 1a, multicohort, dose-escalation trial. Lancet Oncol18: 587-598.
- Ghali F, Gore L, Wright L, Yyu E, Patel D, et al, Surrogate Endpoints as Predictors of Overall Survival in Metastatic Urothelial Cancer: A Trial-level Analysis. Eur Urol Open Sci 47: 58-64.
- Grivas P, Jung S, Cislo P, Chang J, Powles T, et al, (2023) Patient-reported Outcomes from JAVELIN Bladder 100: Avelumab First-line Maintenance Plus Best Supportive Care Versus Best Supportive Care Alone for Advanced Urothelial Carcinoma. Eur Urol 83: 320-328.
- Lalani,AA(2023) How I Do It: Maintenance avelumab for advanced urothelial carcinoma. Can J Urol, 30:11633-11638.
- Maiorano BA, Girogi DA, Schinzari G, Toritora G, Evaristo M et al, (2022) Immune-Checkpoint Inhibitors in Advanced Bladder Cancer: Seize the Day. Biomedicines 10:411.
- Massard C, Gordon S, Saeed R, Sanborn S, Marlon c, et al, (2016) Safety and Efficacy of Durvalumab (MEDI4736), an Anti-Programmed Cell Death Ligand-1 Immune Checkpoint Inhibitor, in Patients With Advanced Urothelial Bladder Cancer. J Clin Oncol 34: 3119-3125.
- Naidoo J, Wang X, Tunc L, Callahan K, Martin V, et al. (2017) Pneumonitis in Patients Treated With Anti-Programmed Death-1/Programmed Death Ligand 1 Therapy. J Clin Oncol 35: 709-717.
- Ten Eyck JE, Sonia M, Hamuda D, Petros G, Kahlon N et al, (2022) Clinical Evaluation of Avelumab in the Treatment of Advanced Urothelial Carcinoma: Focus on Patient Selection and Outcomes. Cancer Manag Res14: 729-738.
- Reits EA, Scholm, Spits H, Mesman E, Arnold H, et al, (2006) Radiation modulates the peptide repertoire, enhances MHC class I expression, and induces successful antitumor immunotherapy. J Exp Med 203: 1259-1271.
- La Rocca E (2018) Radiotherapy with the anti-programmed cell death ligand-1 immune checkpoint blocker avelumab: acute toxicities in triple-negative breast cancer. Med Oncol 36: 4.
- UngaroA, Tucci M, Giorgio V, Massino D, Chiara P, et al. (2002) Antibody-Drug Conjugates in Urothelial Carcinoma: A New Therapeutic Opportunity Moves from Bench to Bedside. Cells 2022. 11(5).
- Hu Z. (2017) The future of immune checkpoint blockade immunotherapy: towards personalized therapy or towards combination therapy. J Thorac Di 9: 4226-4229.
- D’Angelo SP, Lebbe C, Hassel C, Kiecker F, Paul N et al, (2021) First-line avelumab in a cohort of 116 patients with metastatic Merkel cell carcinoma (JAVELIN Merkel 200): primary and biomarker analyses of a phase II study. J Immunother Cancer 9(7).
- Church C, Chan N, Koellhe N, Chan T, Pierce N, et al, (2022) Transcriptional and functional analyses of neoantigen-specific CD4 T cells during a profound response to anti-PD-L1 in metastatic Merkel cell carcinoma. J Immunother Cancer10(9).
- Iacovelli R, Sacco E, Marco R, Bassi F, et al, (2023) Management of metastatic urothelial carcinoma: Current approach, emerging agents, and future perspectives. Urologia 90: 3-10.
- Meeks JJ, Black C, Noah M, Svatek R, Jonathan R et al. (2023) Checkpoint Inhibitors in Urothelial Carcinoma-Future Directions and Biomarker Selection. Eur Urol 84: 473-483.
1* Medical Oncologist, Chief of Genitourinary Tumor Service, Instituto de Oncología “Ángel H. Roffo”. Universidad de Buenos Aires, Av. San Martín 5481. Buenos Aires Argentina
2* Urologist, M.D. Department of Urology. Instituto de Oncología “Ángel H. Roffo”. Universidad de Buenos Aires, Av. San Martín 5481. Buenos Aires Argentina
3* Medical Oncologist, Faculty of Medicine (University of Buenos Aires), Emeritus Member of ASCO, Emeritus Member of ESMO
*Corresponding author: Dr. María Natalia Gandur Quiroga, MD, M.Sc Medical Oncology, Genitourinary Tumor Service, Instituto de Oncología “Ángel H. Roffo”. Universidad de Buenos Aires, Av. San Martín 5481. Buenos Aires Argentina, Tel: +54 (011) 5287-5356; Email: nataliagandur@gmail.com
*Corresponding author: Dr. María Natalia Gandur Quiroga, MD, M.sc Medical Oncology, Genitourinary Tumor Service, Instituto de Oncología “Ángel H. Roffo”. Universidad de Buenos Aires, Av. San Martín 5481. Buenos Aires Argentina, Tel: +54 (011) 5287-5356; Email: nataliagandur@gmail.com
Citation: Dr María Natalia Gandur Quiroga (2023) Avelumab in the Treatment of Bladder Cancer: Current Insights and Future Prospects. Arc Can Res Med 4: 014. DOI: https://doi.org/10.58735/acrmsi114
Received: Aug 21, 2023; Accepted: Oct 05, 2023, Published: Oct 08 2023
Copyright: © 2023 Dr María Natalia Gandur Quiroga. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits un-restricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
