Bilirubin Encephalopathy: A Still Scourge In Morocco
- Article
- Article Info
- Author Info
Introduction
Bilirubin Encephalopathy (BE) is an important cause of morbidity and mortality throughout the world, especially in low-middle-income countries where it accounts for up to 15% of neonatal death. The pathophysiology of this
acute severe disease of infancy and its potential evolution to neurological complications quickly and kernicterus remain poorly understood [1]. Associated risk factors can include a low gestational age, low birth weight, hemolysis, sepsis, cephalohematoma, or easy bruising. Recent literature has shed light on the wide range of clinical symptomatology and pathological findings associated with bilirubin encephalopathy named kernicterus [2-4].
The objectives of our study are to specify the epidemiology of bilirubin encephalopathy and kernicterus in our context, to identify the risk factors and the etiologies in Morocco.
Methods
This is a descriptive and retrospective study. It is carried over 70 months (January 2015 – October 2020) at the neonatal intense care unit of the Mohammed VI University Hospital in Marrakesh. It concerned 40 neonates with hyperbilirubin encephalopathy who were hospitalized for jaundice associated with one or more neurological signs (somnolence, hypotonia, lethargy, opisthotonos, convulsions…). Medical records were analyzed for demographic details of patients (age, sex), clinical and paraclinical aspects, treatments and evolution.
Results
During the study period, 1052 neonates were hospitalized for jaundice, including 40 for bilirubin encephalopathy, which represents 3.8 %. Of these 40 cases, 28 are male (70%) and 12 are female (30%). The sex ratio was 2,33. The age of admission varied between 2 and 19 days with an average of 5.6 days. For the risk factors (Table 1), 18 mothers had a grouping O and 14 had a negative Rhesus, the pregnancy was poorly followed in 50% of cases with a positive anamnesis in 45% of cases, the prematurity was found in 25 % of cases, twenty eight newborns (70%) weigh more than 2000 grams against twelve (30%) whose birth weight is less than 2000 grams.
| Risk Factors | Numbers | Percent (%) |
| Moms blood grouping: | ||
| O+ | 16 | 40 |
| O– | 2 | 5 |
| A+ | 6 | 15 |
| A– | 10 | 25 |
| B+ | 4 | 10 |
| B– | 2 | 5 |
| Abortions or MFIU | 4 | 10 |
| Neonatal death | 8 | 20 |
| Neonatal jaundice in siblings | 10 | 25 |
| Pregnancy monitoring | ||
| Followed / not / poorly followed | 14/ 06/ 20 | 35/ 15/ 50 |
| Infectious history: positive | 18 | 45 |
| Delivery non-medicalized | 10 | 25 |
| Medical delivery | 30 | 75 |
| Vaginal birth / Cesarean | 32/ 08 | 80/ 20 |
| Gestational age: | ||
| prematurity / term | 30 /10 | 25 / 75 |
| Birth weight: | ||
| 800g_2000g | 12 | 30 |
| 2000g_3000g | 16 | 40 |
| 3000g_4000g | 12 | 30 |
| Sero-blood lump | 6 | 15 |
Table 1: Distribution of patients according to risk factors.
A history of neonatal jaundice in siblings was noted in 10 neonates (25% of cases). And there is a history of death in siblings in 20% of cases, the cause of these deaths was not mentioned on the files except in 3 cases where jaundice was reported as the cause. The onset of jaundice was in 30% of cases before 24 hours of life, 55% of newborns presented jaundice between 24h and 72h. While only 15% of cases showed jaundice after the third day of life. The average duration of jaundice before admission was 3 days with extremes ranging from 1 to 17 days. The initial symptomatology was dominated by jaundice (100% of cases) followed by neurological signs (refusal to suckle in 25% of cases and seizures in 20% of cases). Neurological signs were present at admission in 85% of cases dominated mainly by hypotonia and lethargy (Table 2).
| Physical signs | Number of cases | Percent (%) |
| Neurological signs | 32 | 85 |
| Hypotonia or lethargy | 16 | 40 |
| Opisthotonos | 12 | 30 |
| Weak or absent RS | 10 | 25 |
| Weak RA | 4 | 10 |
| Pallor | 5 | 12.5 |
| Dehydration | 2 | 5 |
| Sero-blood lump | 6 | 15 |
Table 2 : Distribution of patients according to clinical signs.
Biologically, total bilirubin at admission ranged from 350 to 1103 µmol/l. Hemolysis was found in 18 patients (45%). The O+ blood group was the most common among mothers followed by Rhesus+ group A. The majority of newborns included in our study had A+ grouping. The direct Coombs test was positive in 12 cases.
The Cytobacteriological Examination of Urine (ECBU) required in 30 neonates and was positive in 16 cases, urinary tract ultrasonography requested in all patients who had a urinary tract infection, did not reveal abnormalities.
Etiologies were dominated by neonatal infection in 40% followed by rhesus incompatibility in 30% and ABO incompatibility in 15% and in 15% of cases the etiology was unknown.
The therapeutic approach of our patients was aimed at an etiological and symptomatic treatment. Thirty-eight newborns received phototherapy (95%), of which 22 were intensive. Two neonates did not receive phototherapy
because they were unstable. Ten neonates were transfused with O negative blood (25%). Sixteen patients received antibiotic treatment (40%).
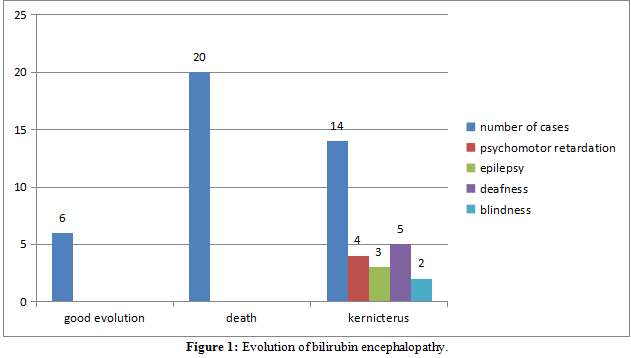
The evolution was, in the short term, good for 06 newborns (15%). We deplored 20 deaths (50%) and the long-term evolution was marked by neurological sequelae (kernicterus) for 14 patients (35%) (Figure 1). The average duration of hospitalization was 4.5 days with extremes of 1 and 17 days.
Discussion
Acute Bilirubin Encephalopathy [BE] is an acute illness caused by severe hyperbilirubinemia. Presenting signs and symptoms include decreased feeding, lethargy, abnormal tone, high-pitched cry, retrocollis and opisthotonus, setting-sun sign, fever, seizures, and possibly death [5,6]. Seizures usually resolve several weeks after the acute insult [4].
Kernicterus also known as chronic bilirubin encephalopathy, describes the chronic, toxic, and permanent sequelae of high levels of unconjugated bilirubin on the central nervous system of infants [7]. A tetrad of clinical features that are typically evident after one year of age: (i) abnormal motor control, movements, and muscle tone; (ii) an auditory processing disturbance with or without hearing loss; (iii) oculomotor impairments, especially impairment of the upward vertical gaze; and (iv) dysplasia of the enamel of deciduous (baby) teeth [4,8].
Globally, bilirubin encephalopathy is one of the most important preventable causes of neonatal mortality and brain injury [9].
Most incidence studies are based on registries of reported cases with sometimes different definitions. In Great Britain, an incidence of 7.1/100,000 cases of indirect hyperbilirubin has been reported ≥ 510 μmol/l [7]. In Denmark, a rate of 45/100,000 ≥ is found in newborns with a total bilirubin level > 450 μmol/l [10]. In both
studies, the kernicterus rate is close to 1/100,000 newborns [10,11]. In Canada, the incidence of acute encephalopathy is 1/49,000 and the incidence of nuclear jaundice is 1/43,000. In the United States, 98/119 newborns (82%) with jaundice had total bilirubinemia ≥ 513 μmol/l [12,13]. In Low Middle Income Countries (LMICs), this is largely due to delayed diagnosis, under-reporting or even lack of organized data collection and reporting in LMICs. [1].
In Morocco, the incidence of neonatal jaundice in general and that of bilirubin encephalopathy remains unknown, due to the lack of a national registry. during our study period bilirubin encephalopathy affected 40 neonates, a frequency of 3.8% of all newborns admitted for neonatal jaundice (1052 neonates) and 14 patients who developed Kernicterus (1.33%).
The precise mechanisms of the toxicity of bilirubin on the brain are still not fully understood. However, all newborns are not equal when it comes to hyperbilinemia [1].
Associated risk factors can include a low gestational age, low birth weight, hemolysis, sepsis, cephalohematoma or easy bruising, and exclusive breast feeding [4]. American Academy of Pediatrics, Subcommittee on Hyperbilirubinemia, 2004, classified the risk factors into major and minor : Major risk factors for severe neonatal hyperbilirubinemia include hemolytic disease, breastfeeding, gestational age less than 38 weeks, significant jaundice in a previous sibling, and an early onset of jaundice. Minor risk factors include maternal diabetes and male sex. Gestational age greater than or equal to 41 weeks, exclusive bottle feeding, African American race, and discharge from the hospital after 72 hours are associated with a decreased risk of developing severe hyperbilirubinemia [9].
Retrospective analysis of the nuclear jaundice registers allowed to identify risk factors for the onset of jaundice severe: gestational age ≤ 37 AS, male, early withdrawal from maternity with bilirubin (or transcutaneous bilirubin) preceding the exit in the risk zone of the predictive curves (normogram), poorly initiated exclusive breastfeeding with loss of weight alloimmunization or ABO group incompatibility, challenge cited in Glucose-6-phosphate dehydrogenase (G6PD) deficiency, East Asian origin, cephalhematoma [10-13].
Acute bilirubin encephalopathy can be subtle requiring a high index of suspicion, or apparent with overt neurologic abnormalities. [1]. Its development is marked by three distinct phases: The first is characterized by poor sucking, hypotonia and weak Moro. The Second phase is hypertonia with extending muscle spasms, opisthotonus, which may alternate with periods of hypotonia, fever and high-pitched cry are often associated. In some cases, we can see food refusal, apnea, stupor or coma and a progression to death. The third hypotonia phase begins at the end of the first week of life In this phase, deep stupor or coma, high pitch crying, pronounced retrocollis-opisthotonos, and no feeding is observed. The clinical features of encephalopathy in preterm neonates are the same as term neonates but more subtle, mainly due to neuronal immaturity and masking clinical conditions [1]. Recognizing these signs early is essential for rapid and appropriate treatment.
Kernicterus is a more chronic and permanent clinical sequel of bilirubin toxicity in neonates who survive Acute bilirubin encephalopathy. In the early phase, which occurs in the first year of life, it usually presents with hypotonia, hyper-reflexia, persistence of tonic neck reflex and delayed milestones [14] After the first year, the manifestation is more variable, with a tetrad of symptoms including auditory, visual and dental abnormalities, and extrapyramidal disturbances. Bulbar functions may also be impacted [1,14].
T.M. Shapiro proposed a classification of the sequelae of acute bilirubin encephalopathy to classical kernicterus;
refers to people with the classic triad or tetrad. Kernicterus predominantly motor and kernicterus with predominance of auditory sequelae [5].
Caffeine is encouraging for the prevention and treatment of bilirubin neurotoxicity in rats due to its anti-apoptotic, antioxidant, anti-inflammatory, anti-nitrosating and anti-TLR-4 properties [15].
For the prevention of kernicterus we have proposed these recommendations:
- Generalization of ABO-RH grouping in all mothers in preconception;
- Monitor breastfed infants closely, support them effectively, and assess them appropriately;
- Ensure a systematic assessment of the risk of severe hyper-bilirubinemia in a newborn;
- Organize appropriate follow-up according to the age of discharge from maternity and the assessment of the risk of severe jaundice;
- Raising awareness among maternity staff of the importance of early detection of jaundice;
- Sensitization of parents and the general public on the seriousness of neonatal jaundice and the value of early treatment.
Conclusion
Severe hyperbilirubinemia accounts for only a small proportion of neonatal jaundice, but they are nevertheless responsible for definitive sequelae (nuclear icterus) in the absence of screening and adapted management. It is a scourge in Morocco given the problem of access to care and the delay in care.
References
- F Usman, UM Diala, SM Shapiro, JB Le Pichon, TM Slusher (2018) Acute bilirubin encephalopathy and its progression to kernicterus: current perspectives. Res Report Neonatol 8: 33-44.
- K Bhardwaj, T Locke, A Biringer, A Booth, EK Darling, et al. (2017) Newborn Bilirubin Screening for Preventing Severe Hyperbilirubinemia and Bilirubin Encephalopathy: A Rapid Review. Curr Pediatr Rev 13: 67-90.
- PW Chang, TB Newman, MJ Maisels (2017) Update on Predicting Severe Hyperbilirubinemia and Bilirubin Neurotoxicity Risks in Neonates. Curr Pediatr Rev 13: 181-187.
- S Das, FKH van Landeghem (2019) Clinicopathological Spectrum of Bilirubin Encephalopathy/Kernicterus. Diagnostics 9: 24.
- SM Shapiro (2013) Bilirubin toxicity in the developing nervous system. Pediatr Neurol 29: 410-421.
- SM Shapiro (2005) Definition of the Clinical Spectrum of Kernicterus and Bilirubin-Induced Neurologic Dysfunction (BIND). J Perinatol 25: 54-59.
- GA Neknek, K Woldemichael, A Moges, D Zewdneh Solomon (2017) MRI of bilirubin encephalopathy (kernicterus): A case series of 4 patients from Sub-Saharan Africa, May 2017. Radiol Case Rep 13: 676-679.
- SM Shaprio (2010) Chronic bilirubin encephalopathy: Diagnosis and outcome. Semin. Fetal Neonatal Med 15: 157-163.
- MF Saavedra, P Kumar (2018) A Case Report of Kernicterus in a Neonatewith Hemolytic Disease of Newborn-Lessons to Learn. J Pediatr Health Care 32: 411-415.
- JV Bjerre, JR Petersen, F Ebbesen (2008) Surveillance of extreme hyperbilirubinaemia in Denmark. A method to identify the newborn infants. Acta Paediatr 97: 1030-1034.
- D Manning, P Todd, M Maxwell, MJ Platt (2007) Prospective surveillance study of severe hyperbilirubinaemia in the newborn in the UK and Ireland. Arch Dis Child Fetal Neonatal Ed 92: F342-F346.
- MJ Maisels (2009) Neonatal hyperbilirubinemia and kernicterus – not gone but sometimes forgotten. Early Hum Dev 85: 727-32.
- A Bedu (2011) Hyperbilirubinémies sévères et ictères nucléaires en France en 2011. Tous droits réservés. Arch de Pédiatrie 18: 17-18.
- M Kaplan, C Hammerman (2005) Understanding severe hyperbilirubinemia and preventing kernicterus: adjuncts in the interpretation of neonatal serum bilirubin. Clin Chim Acta 356: 9-21.
- M Deliktaş, H Ergin, A Demiray, H Akça, OMA Ozdemir, et al. (2019) Caffeine prevents bilirubin-induced cytotoxicity in cultured newborn rat astrocytes. J Matern Fetal Neonatal Med 32: 1813-1819.
1Neonatal Intensive Care Unit, Mother and Child Hospital, Mohammed VI University Hospital, Marrakesh, Morocco
2Laboratory Childhood, Health and Development, Marrakesh Medical School, Cadi Ayyad University, Marrakesh, Morocco
Citation: S. Mrhar, F. Bennaoui, N. El Idrissi Slitine and F. M. R. Maoulainine (2020) Bilirubin Encephalopathy: A Still Scourge In Morocco. Int J Med Surg 1: 001.
Received: Apr 15, 2021; Accepted: May 04, 2021; Published: May 18, 2021
Copyright: © 2021 Soumia Mrhar et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits un-restricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Corresponding Authors:
Soumia Mrhar, Neonatal Intensive Care Unit, Mother and Child Hospital, Mohammed VI University Hospital, Marrakesh 40000, Morocco.
