Melanoma
- Article
- Article Info
- Author Info
Introduction
World Melanoma Day is celebrated on May 23 of each year. Melanoma is the most aggressive type of skin cancer and its incidence has increased significantly in recent decades. This day was established with the aim of raising awareness among the population about the importance of prevention, early detection and appropriate treatment of melanoma [1]
The World Melanoma Day campaign seeks to report on risk factors, the importance of sun protection, skin self-examination and medical consultation for any suspicious changes in moles or skin spots.
The World Melanoma Day was established by the international organization Melanoma World Society (MWS) in 2005. Since then, various organizations, doctors, institutions and communities around the world come together on this date to carry out awareness and education activities about melanoma and its prevention [2,3].
It is important to note that melanoma is a highly dangerous skin cancer, but if it is detected in early stages, it has high cure rates. Therefore, awareness of risk factors, sun protection and early detection are essential to reduce the impact of melanoma on the population
Generalities
Melanoma is a type of cancer that originates in the pigment-producing cells of the skin, known as melanocytes. These cells are responsible for the production of melanin, the pigment that provides color to the skin, hair and eyes. Melanoma can develop in both the skin and mucous membranes, such as the membranes that cover the digestive tract, urinary tract and reproductive organs.
Melanoma can affect people of all ages, but it is more common in young adults. Although it is less common than other types of skin cancer, such as basal cell carcinoma or squamous cell carcinoma, melanoma is more aggressive and has a greater potential for spread to other parts of the body if it is not detected and treated in time [4,5].
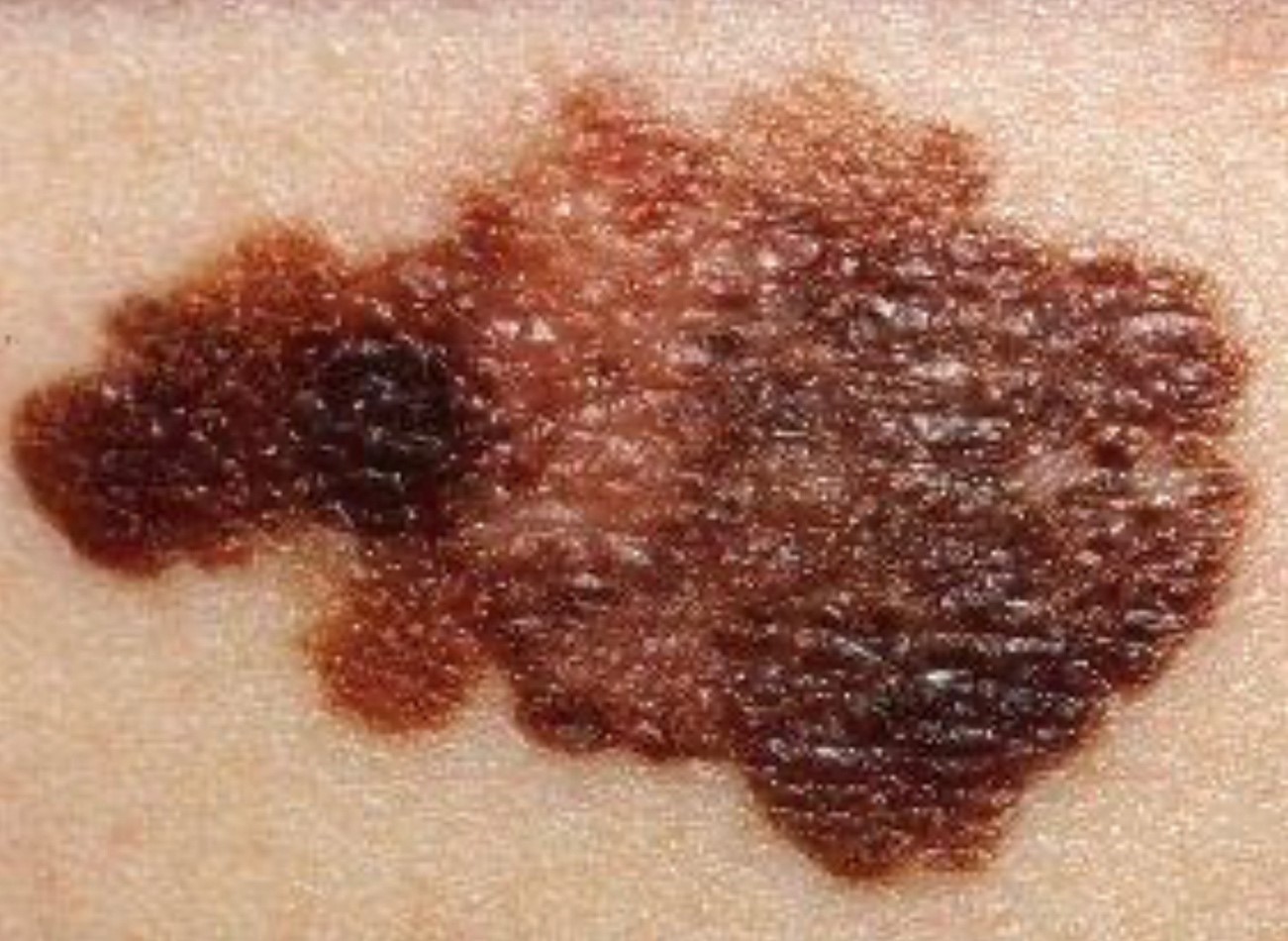
The signs and symptoms of melanoma may vary, but they are usually characterized by changes in the appearance of moles or the appearance of new skin lesions. Some of the warning signs of melanoma include:
Asymmetry: a mole or skin lesion with an irregular or asymmetrical shape.
Irregular edges: the edges of a mole or injury are uneven, jagged or blurred.
Variable coloration: a mole or an injury that has different shades of color, such as black, brown, blue, red or white.
Diameter: a mole or an injury with a diameter greater than 6 mm (although it may also be smaller).
Evolution: changes in the size, shape, color or texture of an existing mole, or the sudden appearance of a new injury.
How does Melanoma develop?
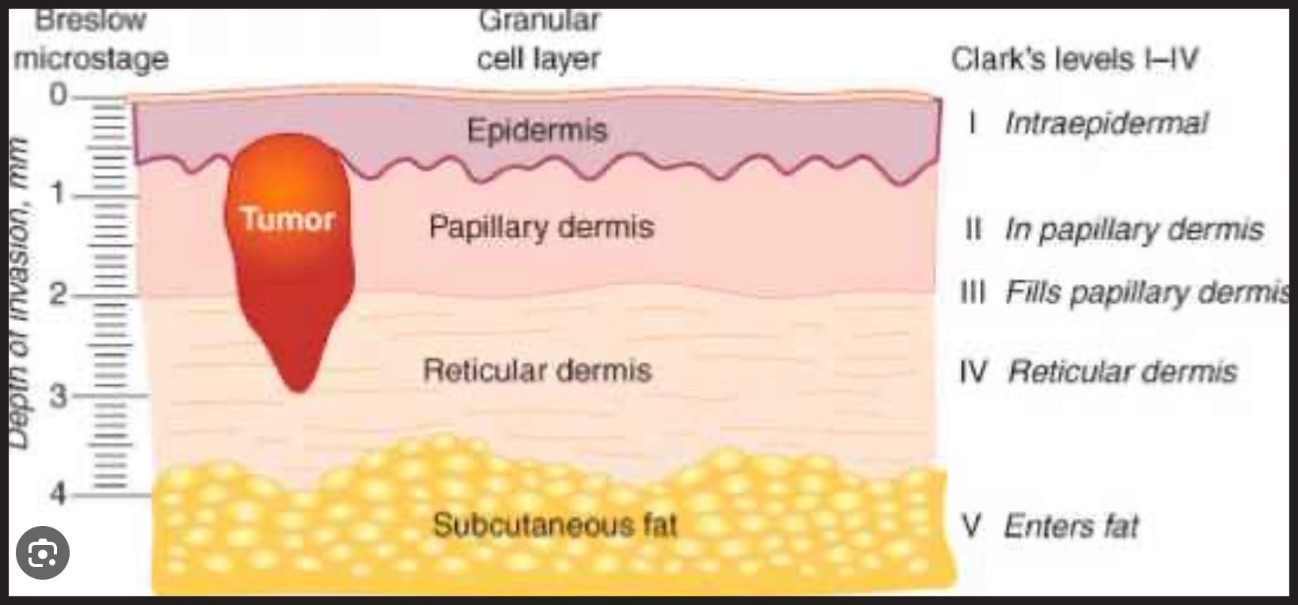
Clark Levels and Breslow Depth
Clark levels and Breslow depth are two classification systems used to evaluate the extent of melanoma and determine its staging. Both systems provide important information about the invasion of melanoma in the skin and are used to guide treatment and predict the prognosis of the disease.
Clark levels: Clark levels classify the invasion of melanoma in the skin into five stages, from level I to level V. Each level is defined according to the depth of invasion and the layer of the affected skin. Clark’s levels are described below:
Level I: Melanoma is found only in the upper layer of the epidermis (the outermost layer of the skin).
Level II: Melanoma invades the epidermis until the junction between the epidermis and the dermis (the middle layer of the skin).
Level III: Melanoma extends through the junction between the epidermis and the dermis and is located in the upper part of the dermis.
Level IV: Melanoma invades the dermis in its entirety.
Level V: Melanoma extends beyond the dermis and affects deeper tissues, such as subcutaneous tissue, muscles or bones.
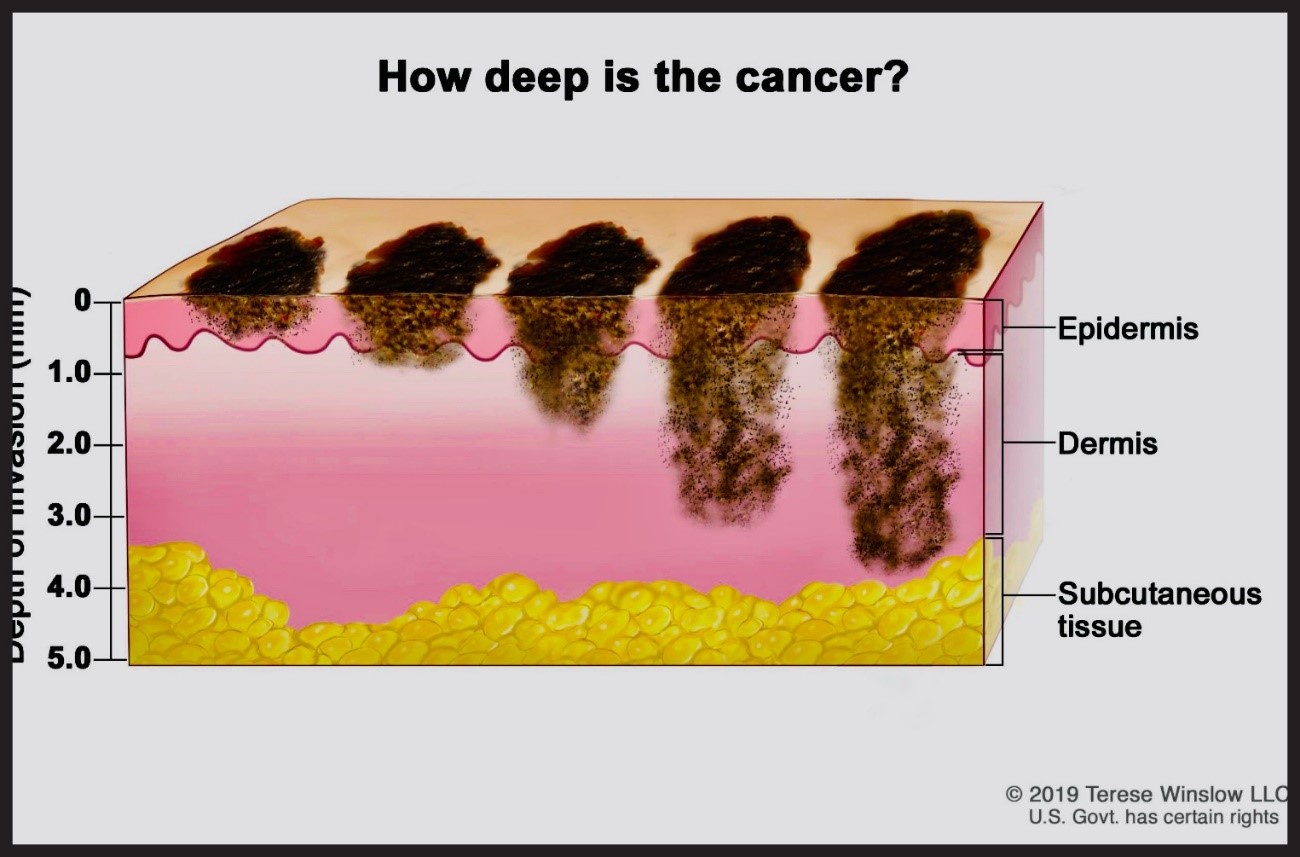
Breslow depth: The Breslow depth measures the vertical distance in millimeters from the most superficial layer of the epidermis to the deepest point of melanoma invasion. It provides information about the thickness of melanoma and its ability to spread. The greater the depth of Breslow, the greater the risk of metastasis. It is accurately measured using a microscope to examine a melanoma biopsy.
Both classification systems, the Clark levels and the Breslow depth, are important for determining the stage of melanoma and planning the appropriate treatment. Other factors, such as the presence of metastases in the lymph nodes and the presence of cancer cells in the bloodstream, are also taken into account for staging and the prognosis of melanoma.
Molecular Biology and Genetic Factors
Molecular biology refers to the study of biological processes at the molecular level, focusing on cellular components, such as nucleic acids (DNA and RNA), proteins and the interactions between them. Molecular biology has revolutionized our understanding of genetics, cell function and the underlying mechanisms of various diseases, including skin cancer such as melanoma [6-8].
In the case of melanoma, it has been discovered that several genetic factors play an important role in the development and progression of the disease. Some of the known genetic factors include:
Mutations in tumor suppressor genes: Mutations in tumor suppressor genes have been identified, such as the p53 gene and the CDKN2A gene (which encodes the p16INK4a protein), in some cases of melanoma. These mutations can predispose cells to uncontrolled proliferation and tumor formation.
Mutations in cell cycle regulatory genes: Alterations in genes that regulate the cell cycle, such as the BRAF and NRAS genes, are common in melanoma. These mutations can activate cell signaling pathways that promote the growth and survival of cancer cells.
Molecular markers: Several molecular markers associated with melanoma have been identified, such as the expression of the S100 protein, the epidermal growth factor receptor (EGFR) and the platelet-derived growth factor receptor (PDGFR). These markers can help doctors diagnose melanoma and determine the prognosis of the disease [9].
Genomic instability: Melanoma often shows genomic instability, which means that there is a high rate of mutations and genetic changes in tumor cells. This may be the result of defects in DNA repair mechanisms, which allows the accumulation of mutations and the progression of cancer.
Understanding the molecular biology and genetic factors of melanoma is crucial for the development of targeted therapies and personalized treatment approaches. The identification of specific mutations and other molecular biomarkers can help guide treatment and improve results for patients with melanoma [10,11].
Treatments
The treatment of melanoma can vary depending on the stage of the disease, the location and extent of the tumor, as well as the individual characteristics of the patient. Below are some common treatment options used for melanoma:
Surgery: Surgery is the main treatment for melanoma. Depending on the stage of melanoma, it may involve the removal of melanoma along with a margin of surrounding healthy skin. In more advanced cases, it may be necessary to remove the nearby lymph nodes affected by metastasis. In some select cases, reconstructive surgery can be considered after the removal of melanoma.
Immunotherapy: Immunotherapy is a treatment approach that stimulates the body’s immune system to fight melanoma. It may include the administration of medications such as immune checkpoint inhibitors (such as ipilimumab or pembrolizumab) that help unblock the immune system’s defenses against cancer cells [12].
Targeted therapy: Some melanomas have specific genetic mutations that may be targeted in targeted therapies. These medications (such as vemurafenib or dabrafenib) are designed to block or inhibit abnormal signaling pathways that promote melanoma growth.
Chemotherapy: Although melanoma is generally not very sensitive to conventional chemotherapy, in cases of advanced or metastatic melanoma, its use can be considered in combination with other treatments. However, other treatment approaches, such as immunotherapy and targeted therapy, have been shown to be more effective in many cases.
Radiation therapy: Radiation therapy uses high-energy radiation to destroy cancer cells or reduce their growth. It can be used after surgery to destroy residual cancer cells or in cases of advanced melanoma to relieve symptoms and control tumor growth.
The treatment of melanoma is highly individualized and requires the evaluation of a specialized medical team. The oncologist or dermatologist will work together with the patient to determine the most appropriate treatment approach based on the stage of melanoma, general health status and other relevant factors [13].
Role of the Sentinel Ganglion
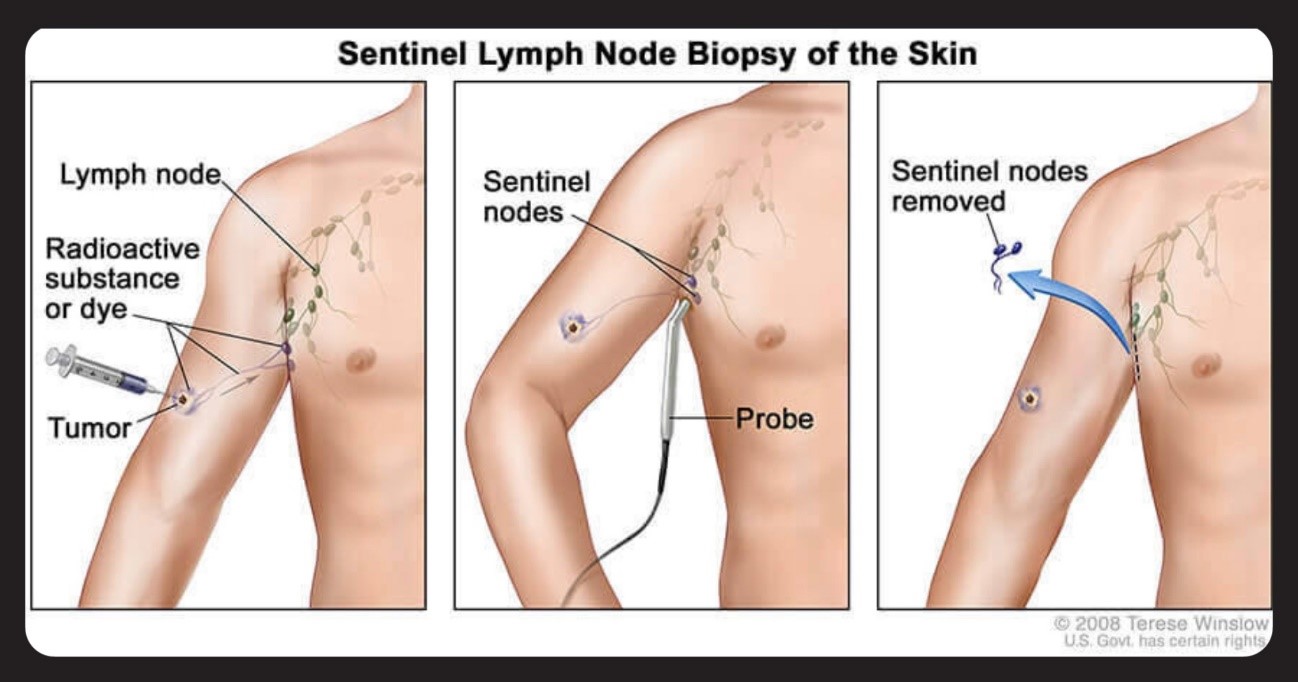
The sentinel ganglion is the first lymph node to which cancer cells are normally directed if they spread from a primary tumor, such as melanoma. This concept is important in the staging and treatment of cancer, including melanoma.
The sentinel node procedure involves the identification and surgical removal of the lymph node that is closest to the site of the primary tumor. It is considered the “guardian” or the first point of lymphatic drainage from the tumor. If the sentinel node shows signs of cancer, it is likely that nearby lymph nodes will not be affected either, thus avoiding more invasive surgery to remove all lymph nodes in the región [14].
The sentinel ganglion procedure usually involves the following steps:
Injection of a dye or a radioactive marker: A dye or a radioactive marker is injected near the primary tumor. This can be done in the operating room or in a pre-surgery session.
Sentinel ganglion tracking: The dye or radioactive marker travels through the lymphatic system to the sentinel ganglion. Surgeons use a special probe or a gamma chamber to locate the sentinel ganglion.
Removal of the sentinel ganglion: Once located, a surgical incision is made to remove the sentinel ganglion. This sample is sent to the laboratory for pathological analysis.
Pathological analysis: Pathologists examine the sentinel ganglion for the presence of cancer cells. If no cancer cells are found, it is considered that nearby lymph nodes are not affected.
The sentinel ganglion procedure is a standard technique for determining the spread of cancer in melanoma and other types of cancer, such as breast cancer and head and neck cancer. It helps doctors to plan the appropriate treatment and determine the patient’s prognosis
Biological Therapies
Biological therapies, also known as directed therapies or directed biological therapies, are a type of treatment that focuses on blocking or interfering with specific molecular pathways involved in the growth and spread of cancer. These therapies are developed from the knowledge of molecular biology and the genetic factors of cancer, including melanoma [15].
In the case of melanoma, several targeted biological therapies have been developed that have been shown to be effective in specific subgroups of patients with particular genetic mutations. Some of the biological therapies used in the treatment of melanoma include:
BRAF inhibitors: Mutations in the BRAF gene occur in approximately half of melanoma cases. BRAF inhibitors, such as vemurafenib and dabrafenib, block the activity of the mutated BRAF protein, which is responsible for the activation of the MAP kinase signaling pathway. These inhibitors have been shown to be effective in the treatment of melanomas with BRAF mutations.
MEK inhibitors: In combination with BRAF inhibitors, MEK inhibitors, such as trametinib and cobimetinib, can be used. These drugs block an enzyme called MEK, which is located downstream of BRAF in the signaling pathway. The combination of BRAF and MEK inhibitors can improve the results in patients with melanoma with BRAF mutations.
Immunotherapy: Immunotherapy is another form of biological therapy that uses the body’s immune system to fight cancer. Immune control point inhibitors, such as ipilimumab and pembrolizumab, are used in the treatment of advanced or metastatic melanoma. These drugs block proteins that regulate the immune response and help activate the immune system to attack cancer cells [16].
Therapies in Research
Advanced immunotherapy: New immunotherapy therapies for melanoma are being researched and developed. This includes the development of more specific and effective immune control point inhibitors, as well as combinations of different immunotherapies to improve results in patients.
Targeted therapies: New targeted therapies are being explored for specific subgroups of patients with particular genetic mutations in melanoma. Molecular signaling inhibitors and key signal transduction pathways are being identified and developed to block the growth and spread of melanoma.
Adoptive cell therapy: Adoptive cell therapy involves the genetic modification of the cells of the patient’s immune system so that they are more effective in the detection and destruction of cancer cells. Studies are being carried out to investigate the efficacy and safety of adoptive cell therapy in the treatment of melanoma.
Therapeutic vaccines: Therapeutic vaccines are designed to stimulate the body’s immune system to recognize and attack cancer cells. Different types of vaccines are being investigated, such as peptide vaccines, dendritic cell vaccines and DNA vaccines, for the treatment of melanoma.
Photodynamic therapy: Photodynamic therapy uses a combination of a photosensitizing agent and light to destroy cancer cells. Studies are being carried out to evaluate the effectiveness of photodynamic therapy in the localized treatment of melanoma.
Genetic therapy: Gene therapy approaches for melanoma are being investigated, which involve the introduction of specific genes into cancer cells to inhibit their growth or induce their death. This includes the development of modified viral vectors to deliver selective therapeutic genes to melanoma.
Combined therapy: Combinations of different therapeutic approaches are being explored, such as the combination of immunotherapy with targeted therapies or with conventional therapies, such as chemotherapy. The goal is to improve the results and overcome the resistance to treatment observed in some cases [17].
Predictive and diagnostic biomarkers: Researchers are working on the identification and validation of biomarkers that can predict the response to treatment and prognosis in patients with melanoma. These biomarkers could help customize therapeutic approaches and improve the accuracy of the diagnosis.
Therapy based on epigenetics: Epigenetics refers to changes in gene expression that do not imply changes in the DNA sequence. Epigenetic alterations in melanoma are being studied and therapies are being developed that aim to correct or reverse these changes, thus restoring the normal function of tumor suppressor genes.
Prevention and early detection studies: The research also focuses on melanoma prevention strategies, such as the identification of modifiable risk factors and the development of more effective sun protection measures. In addition, early detection methods, such as blood tests and imaging tests, are being evaluated to improve the detection and early diagnosis of melanoma.
Suggested Therapeutic Algorithm for Melanoma
Diagnosis of melanoma: A biopsy of the suspected melanoma lesion is performed to confirm the diagnosis. The stage of melanoma is determined according to the depth of Breslow, the invasion of the tissue and the presence of metastases.
Primary surgery: Surgical removal of primary melanoma is performed and the presence of involvement of the margins and nearby lymph nodes is evaluated. In some cases, it may be necessary to perform margin enlargement surgery or sentinel ganglion surgery.
Molecular analysis: Molecular tests are performed to identify specific genetic mutations, such as mutations in the BRAF gene. This helps to determine the most appropriate targeted treatment, if necessary.
Staging and risk assessment: A complete evaluation of the extent of melanoma is carried out and the risk of recurrence and metastasis is determined. This may include imaging tests, such as computed tomography or magnetic resonance imaging, and lymph node analysis.
Adjuvant therapy: In patients with melanoma at high risk of recurrence, adjuvant therapy can be considered to reduce the likelihood of relapse. This may include immunotherapy or targeted therapy, depending on the characteristics of the melanoma and the genetic mutations present.
Systemic therapy for advanced melanoma: In cases of advanced or metastatic melanoma, systemic therapy is considered. This may include immunotherapy with immune checkpoint inhibitors or targeted therapy with BRAF/MEK inhibitors, depending on the molecular characteristics of the tumor [18].
Follow-up and monitoring: Regular follow-up of the patient is carried out with physical examinations, blood tests and imaging tests to detect the recurrence or progression of melanoma. Follow-up also involves monitoring the general health and educating the patient about the early detection of new signs or symptoms.
Prognosis and Survival
The prognosis and five- and ten-year survival for melanoma depend on several factors, including the stage at which melanoma is diagnosed, the thickness of the tumor, the presence of metastases and other individual factors of the patient. It is important to remember that these are only general data and that each case is unique. In addition, advances in diagnosis and treatment can influence survival rates in the future. Below is an overview of the five- and ten-year survival for melanoma according to the stage [19]:
Stage 0 melanoma (in situ melanoma): In general, the five- and ten-year survival for stage 0 melanoma is very high, exceeding 95% in most cases. This is because stage 0 melanoma is found only in the outermost layers of the skin and has not invaded deeper tissues or spread to the lymph nodes or other organs.
Stage I melanoma: Stage I melanoma is characterized by having a Breslow thickness of less than 1 mm and does not present ulceration. The five-year survival for stage I melanoma varies between 85% and 95%, depending on factors such as tumor thickness and the presence of ulceration. The ten-year survival is also high, but it can decrease slightly.
Stage II melanoma: Stage II melanoma is subdivided into stage IIA, IIB and IIC, depending on the thickness of the tumor and the presence of ulceration and involvement of the lymph nodes. The five-year survival for stage II melanoma varies between 50% and 85%, depending on the substage and other risk factors. The ten-year survival may be slightly lower.
Stage III melanoma: Stage III melanoma is characterized by the involvement of regional lymph nodes and, in some cases, the spread to nearby areas of the skin. The five-year survival for stage III melanoma varies widely, from about 40% to about 70%, depending on the number of nodes affected and the extent of the disease. Ten-year survival can also be variable.
5. Stage IV melanoma: Stage IV melanoma refers to metastatic melanoma, where cancer has spread to distant organs, such as the liver, lungs, brain or other sites. The five-year survival for stage IV melanoma is lower, usually about 10% to 15%. However, advances in targeted therapy and immunotherapy have significantly improved treatment options and survival for some patients with metastatic melanoma?
References
- Adrian Pablo Hunis (2020) MELANOMA -Diagnosis, Pathology and Molecular Biology WHAT HAS ASCO 2020 Canc Therapy & Oncol Int J 17: 555952.
- Luke JJ, Flaherty KT, Ribas A, Long GV (2017) Targeted agents and immunotherapies: optimizing outcomes in melanoma. Nat Rev Clin Oncol 14: 463-482.
- Eggermont AM, Spatz A, Robert C (2014) Cutaneous melanoma. Lancet. 2014;383(9919):816-827.
- Larkin J, Chiarion-Sileni V, Gonzalez R,Grob JJ, Rutkowski P (2019) Five-Year Survival with Combined Nivolumab and Ipilimumab in Advanced Melanoma. N Engl J Med 381: 1535-1546.
- Hauschild A, Grob JJ, Demidov LV, Jouary T, Ralf G (2012) Dabrafenib in BRAF-mutated metastatic melanoma: a multicentre, open-label, phase 3 randomised controlled trial. Lancet. 380: 358-365.
- Long GV, Hauschild A, Santinami M, Hodi FS, Mandala M et al. (2017) Adjuvant Dabrafenib plus Trametinib in Stage III BRAF-Mutated Melanoma. N Engl J Med 377: 1813-1823.
- Weber JS, D’Angelo SP, Minor D, Gutzmer R, Neyns B et al. (2015) Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): a randomised, controlled, open-label, phase 3 trial. Lancet Oncol 16: 375-384.
- Ascierto PA, Del Vecchio M, Robert C, Mackiewicz A, Arance A et al. (2017) Ipilimumab 10 mg/kg versus ipilimumab 3 mg/kg in patients with unresectable or metastatic melanoma: a randomised, double-blind, multicentre, phase 3 trial. Lancet Oncol 18: 611-622.
- Schadendorf D, Hodi FS, Robert C, Weber JS, Margolin K et al. (2015) Pooled analysis of long-term survival data from phase II and phase III trials of ipilimumab in unresectable or metastatic melanoma. J Clin Oncol 33:1889-1894.
- Sullivan RJ, Flaherty KT (2013) Resistance to BRAF-targeted therapy in melanoma. Eur J Cancer. 49:1297-1304.
- Eggermont AM, Suciu S, Santinami M, Testori A, Wim HJ et al. (2008) Adjuvant therapy with pegylated interferon alfa-2b versus observation alone in resected stage III melanoma: final results of EORTC 18991, a randomised phase III trial. Lancet 372:117-126.
- Robert C, Karaszewska B, Schachter J, Rutkowski P, Andrzej M et al. (2015) Improved overall survival in melanoma with combined dabrafenib and trametinib. N Engl J Med 372: 30-39.
- Long GV, Stroyakovskiy D, Gogas H, Levchenko E, de Braud F et al. (2015) Dabrafenib and trametinib versus dabrafenib and placebo for Val600 BRAF-mutant melanoma: a multicentre, double-blind, phase 3 randomised controlled trial. Lancet 386: 444-451.
- Sosman JA, Kim KB, Schuchter L (2012) SSurvival in BRAF V600-mutant advanced melanoma treated with vemurafenib. N Engl J Med 366: 707-714.
- Johnson DB, Puzanov I (2015) Treatment of NRAS-mutant melanoma. Curr Treat Options Oncol. 16: 15.
- Dummer R, Hauschild A, Lindenblatt N, O Michielin, Keilholz U et al. (2015) Cutaneous melanoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 5:v126-v132.
- Gyorki DE, Busam K (2015) Diagnosis and management of melanoma. Surg Oncol Clin N Am. 24:197-211.
- Long GV, Eroglu Z, Infante J, Sapna P, Daud A (2018) et al. Long-term outcomes in patients with BRAF V600-mutant metastatic melanoma who received dabrafenib combined with trametinib. J Clin Oncol 36: 667-673.
- Sullivan RJ, Fisher DE (2014) Understanding the biology of melanoma and therapeutic implications. Hematol Oncol Clin North Am 28: 437-453.
Adrián Pablo Hunis
*Member Emeritus of ASCO, Emeritus Member of ESMO
*Corresponding author: Dr. Adrian P. Hunis, Member Emeritus of ASCO, Emeritus Member of ESMO. E-mail: aphunis@gmail.com
Citation: Dr Adrian P. Hunis (2023) Melanoma. Arc Can Res Med 4: 010.
Received: May 16, 2023; Accepted: May 24, 2023, Published: May 28, 2023
Copyright: © 2023 Dr Adrian P. Hunis. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits un-restricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
