Triple-Negative Breast Cancer (TNBC)
- Article
- Article Info
- Author Info
Introduction
Triple-negative breast cancer (TNBC) is a subtype of breast cancer that lacks expression of estrogen receptor, progesterone receptor, and human epidermal growth factor receptor 2 (HER2). It represents a challenging form of breast cancer due to limited treatment options and higher risk of recurrence. Research efforts are ongoing to better understand TNBC’s biology and to develop targeted therapies for improved patient outcomes [1].
Definition, epidemiological and statistical data
Triple-negative breast cancer (TNBC) is a specific subtype of breast cancer characterized by the absence of three important receptor proteins: estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2). This means that TNBC does not respond to hormonal therapies or targeted treatments that target these receptors, making it more difficult to treat compared to other types of breast cancer [2,3]
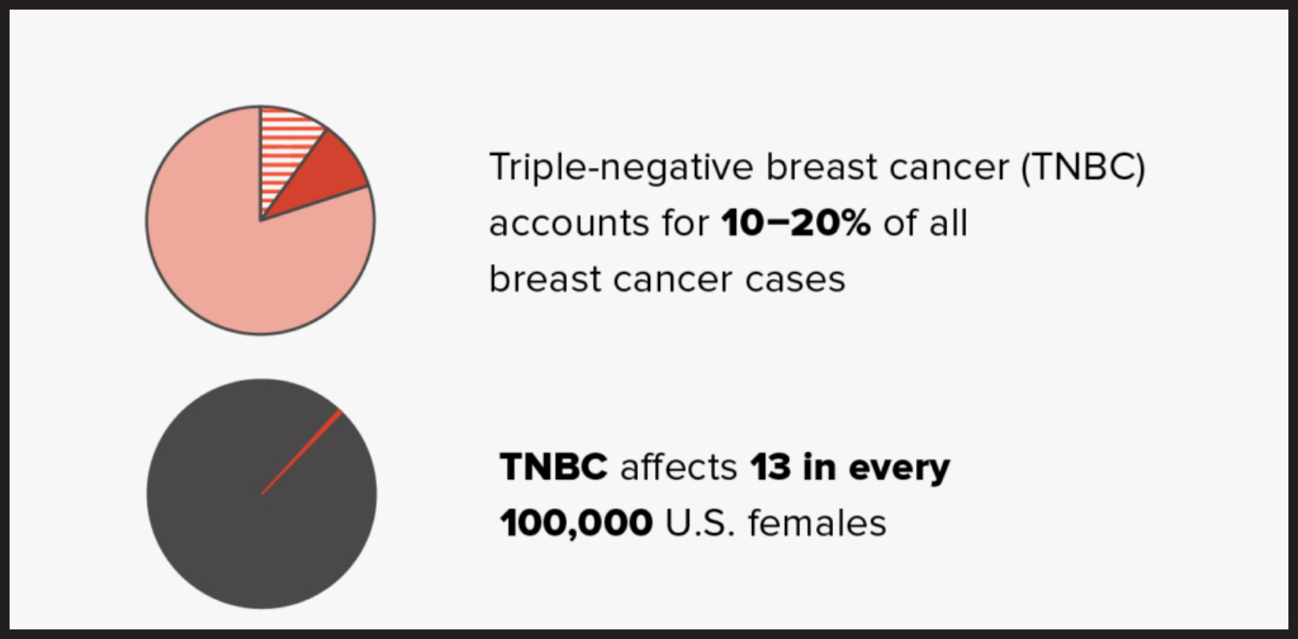
Epidemiological and statistical data for TNBC can vary based on geographical location, race, and other factors.TNBC was estimated to account for approximately 10-20% of all breast cancer cases. It tends to affect younger women more frequently and is more common in African American and Hispanic populations compared to Caucasians [Figure 1].
The incidence of TNBC is relatively higher in premenopausal women, individuals with a family history of breast cancer, and those who carry the BRCA1 gene mutation. It also has a higher incidence in certain countries or regions with specific risk factors.
Classification based on molecular data. Pathological anatomy. Molecular biology and genetic data
Triple-negative breast cancer (TNBC) can be classified based on molecular data, pathological anatomy, molecular biology, and genetic data. Here’s an overview:
Classification based on Molecular Data:
TNBC can be classified into different molecular subtypes based on gene expression patterns. Common subtypes include basal-like, mesenchymal, and claudin-low. These subtypes have distinct molecular characteristics and may have varying responses to treatments.
Pathological Anatomy:
Pathological examination of TNBC tumors reveals specific characteristics. Histologically, TNBC tumors often exhibit high-grade features, prominent lymphocytic infiltrates, and medullary-like patterns. They tend to have a high proliferation rate, which contributes to their aggressive behavior.
Molecular Biology:
TNBC is characterized by the absence of three important receptors: estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2). Without these receptors, targeted therapies that rely on these receptors are not effective in treating TNBC [4, 5].
Genetic Data:
Researchers have identified various genetic alterations associated with TNBC. Mutations in the BRCA1 gene are frequently found in TNBC cases. Other genetic alterations, such as TP53 mutations and PIK3CA mutations, may also play a role in TNBC development.
Understanding the molecular and genetic characteristics of TNBC is crucial for developing targeted therapies and improving treatment outcomes for patients. Ongoing research continues to shed light on the complex biology of TNBC, aiming to find more effective treatment strategies for this aggressive subtype of breast cancer.
Triple-negative breast cancer (TNBC) tends to be more common in younger women compared to older women. It has a predilection for affecting women under the age of 50. However, it’s important to note that breast cancer, in general, is more commonly diagnosed in older women.
Regarding race, TNBC is more frequently diagnosed in African American and Hispanic women compared to Caucasian women. Studies have shown that African American women have a higher incidence of TNBC compared to other racial groups [Figure 2].

It’s essential to remember that breast cancer is a complex disease influenced by various factors, including genetics, lifestyle, and environmental exposures. While these trends may be observed in certain populations, individual cases can vary, and anyone, regardless of age or race, can be affected by TNBC
The aggressive behavior of triple-negative breast cancer (TNBC) is primarily due to the absence of three receptors: estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2). These receptors play important roles in cell growth and division, and their absence in TNBC cells means that hormone-based therapies and targeted treatments that rely on these receptors are not effective. As a result, TNBC tends to grow and spread more rapidly than other types of breast cancer [6].
Regarding the percentage of women who may have TNBC, it represents approximately 15-20% of all breast cancer cases. It’s important to understand that breast cancer is a complex disease, and the incidence of TNBC can vary based on factors such as age, race, and geographical location.
As for men, while breast cancer is much rarer in males, it is possible for them to develop TNBC. Although the vast majority of breast cancer cases occur in women, men can have breast tissue that can undergo malignant transformation. Men with breast cancer may also be classified into different subtypes, including TNBC, based on the presence or absence of hormone receptors and HER2.
Overall, TNBC’s aggressive behavior and occurrence in both women and men emphasize the need for early detection, increased awareness, and ongoing research to improve treatment options and outcomes for all affected individuals. Regular screenings and seeking medical attention for any breast changes are crucial in detecting breast cancer at its early stages when treatment can be more effective.
Genetics and Molecular Findings in Triple-Negative Breast Cancer (TNBC)
Genetics:
1.BRCA1 Mutation:
One of the most well-known genetic factors associated with TNBC is the BRCA1 gene mutation. Women with inherited BRCA1 mutations have a significantly higher risk of developing TNBC. BRCA1 is involved in DNA
repair, and when this gene is mutated, it can lead to an increased susceptibility to breast cancer, particularly the TNBC subtype.
2.TP53 Mutation:
Another common genetic alteration in TNBC is mutations in the TP53 gene. TP53 is a tumor suppressor gene responsible for preventing the growth of abnormal cells. Mutations in TP53 can lead to uncontrolled cell growth and are associated with aggressive cancer behavior.
3.Other Genetic Alterations:
Besides BRCA1 and TP53 mutations, TNBC may harbor various other genetic changes that contribute to its development and progression. These alterations can involve genes related to cell cycle regulation, DNA repair, and cell signaling pathways.
Molecular Findings:
1. Basal-like Subtype:
The majority of TNBC cases belong to the basal-like molecular subtype. Basal-like TNBC tumors express genes similar to basal cells found in the outer layer of the breast ducts. This subtype is often associated with aggressive behavior and poorer prognosis.
2. Immune Response:
TNBC tumors are known for their higher levels of tumor-infiltrating lymphocytes (TILs) compared to other breast cancer subtypes. The presence of TILs is indicative of an immune response against the tumor, and it has been linked to better outcomes in some cases.
3. Androgen Receptor Expression:
A subset of TNBC cases may express the androgen receptor (AR). AR-positive TNBC tumors have shown potential for targeted therapies using anti-androgen drugs [7, 8].
Understanding the genetics and molecular characteristics of TNBC is crucial for developing personalized treatment approaches. Researchers are continually investigating these findings to identify potential therapeutic targets and improve patient outcomes. Additionally, ongoing research may lead to the development of novel therapies tailored to specific genetic subtypes of TNBC.
Signs and Symptoms of TNBC
A TNBC clinic is a medical facility or department that specializes in diagnosing, treating, and managing patients with triple-negative breast cancer (TNBC). These clinics typically have a multidisciplinary team of healthcare professionals, including oncologists, surgeons, radiologists, pathologists, and nurses, working together to provide comprehensive care to patients with this aggressive breast cancer subtype.
Signs and Symptoms of TNBC:
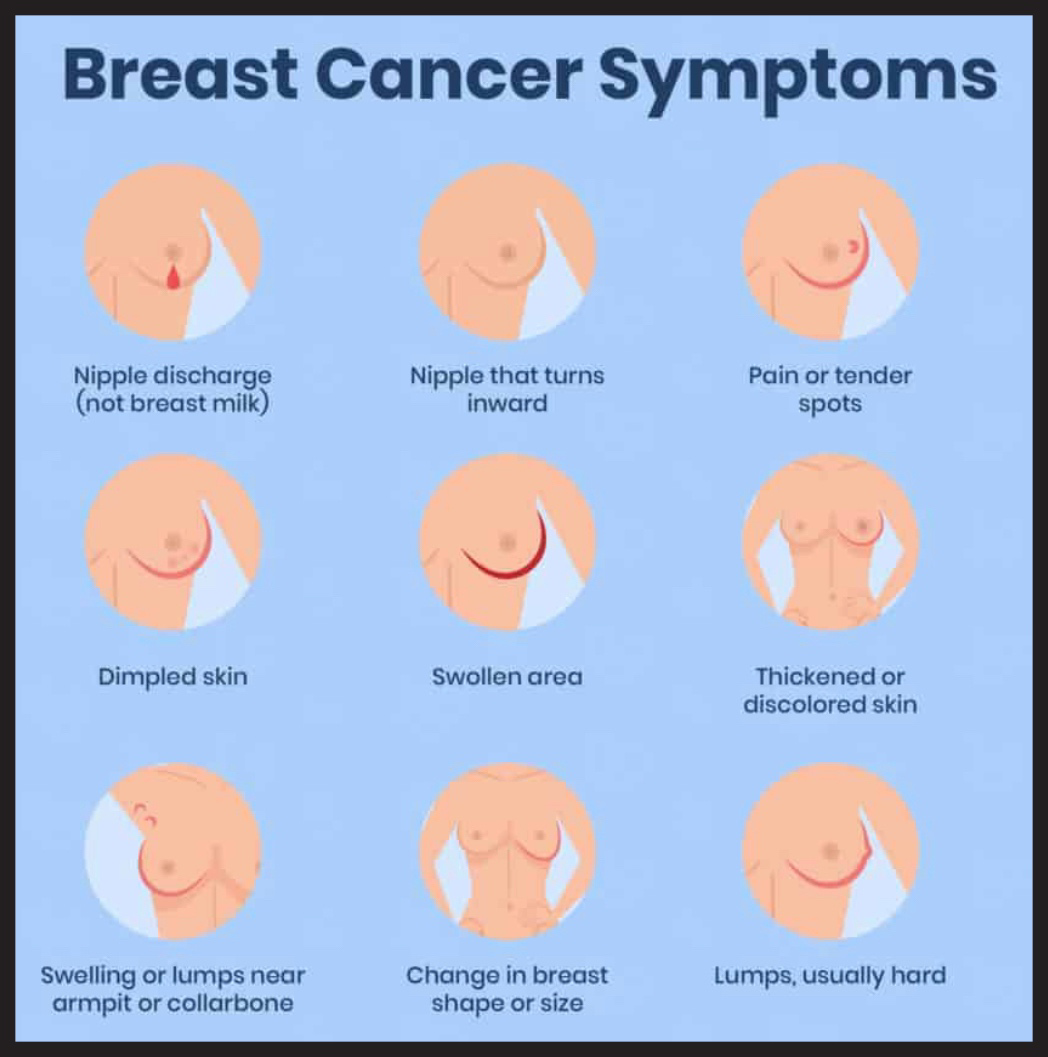
The signs and symptoms of TNBC are similar to those of other types of breast cancer. Some common signs to look out for include:
- A lump or thickening in the breast or underarm area.
- Breast pain or tenderness.
- Skin changes on the breast, such as redness, dimpling, or puckering.
- Nipple changes, such as inversion, scaling, or discharge (other than breast milk).
- Swelling or changes in the size or shape of the breast.
Diagnosis
Diagnosing triple-negative breast cancer (TNBC) involves a combination of imaging studies, laboratory tests, and tissue analysis. Here’s an overview of the diagnostic process:
1.Clinical Evaluation:
The process usually begins with a clinical evaluation by a healthcare provider, who will inquire about any symptoms or changes in the breasts and perform a physical examination.
2.Imaging Studies:
a. Mammogram: A mammogram is a standard imaging test for breast cancer screening. It uses X-rays to detect abnormalities in the breast tissue, such as lumps or calcifications.
b. Ultrasound: Breast ultrasound may be used to further evaluate suspicious areas detected on a mammogram. It helps distinguish between fluid-filled cysts and solid masses.
c. Magnetic Resonance Imaging (MRI): Breast MRI is often used in specific cases, such as when there are high-risk factors or to assess the extent of the disease in the breast [9].
3.Biopsy:
If imaging tests reveal a suspicious mass or abnormality, a biopsy is performed to obtain a sample of the breast tissue for analysis. There are different types of biopsies, including:
a. Core Needle Biopsy: A small tissue sample is obtained using a thin needle.
b. Fine Needle Aspiration (FNA): A thin needle is used to aspirate cells from a lump or mass.
c. Surgical Biopsy: In some cases, a surgical procedure may be necessary to remove a larger tissue sample for examination.
4. Pathological Analysis: The biopsy sample is sent to a pathology laboratory, where a pathologist examines the tissue under a microscope to determine if cancer is present. If cancer is identified, further testing is performed to characterize the tumor, including determining hormone receptor status (ER, PR), HER2 status, and Ki-67 proliferation index.
5.Laboratory Studies:
a. Hormone Receptor and HER2 Testing: Immunohistochemistry (IHC) and/or fluorescence in situ hybridization (FISH) are used to determine the expression of hormone receptors (ER, PR) and the HER2 protein.
b. Ki-67 Proliferation Index: Ki-67 staining is used to assess the tumor’s growth rate and help determine its aggressiveness [10, 11].
Most Frequent Metastatic Dissemination Patterns
Triple-negative breast cancer (TNBC) has distinct metastatic dissemination patterns that commonly involve certain organs. The most frequent sites of metastasis in TNBC include:
- Lungs: The lungs are one of the most common sites of metastasis in TNBC. Cancer cells can spread to the lungs through the bloodstream, forming secondary tumors.
- Liver: Metastases to the liver are also common in TNBC. Cancer cells can travel through the bloodstream and establish secondary tumors in the liver.
- Brain: TNBC has a higher propensity for metastasizing to the brain compared to other breast cancer subtypes. Cancer cells can cross the blood-brain barrier and form brain metastases.
- Bones: Bone metastases are common in many types of advanced breast cancer, including TNBC. Cancer cells can invade the bones, causing pain, fractures, and other skeletal-related issues.
- Lymph Nodes: Lymph nodes in the axilla (underarm), as well as nodes in other regions of the body, can be affected by TNBC metastases.
It’s important to note that while these sites are more frequently involved in metastatic TNBC, cancer cells can potentially spread to other organs as well. The risk and pattern of metastasis can vary based on the tumor’s stage, grade, and other factors. Detecting and monitoring metastases is essential for managing advanced TNBC and determining appropriate treatment strategies.
Staging
For staging and determining the extent of triple-negative breast cancer (TNBC), various diagnostic tests and imaging studies are commonly used. The combination of these tests helps oncologists and healthcare providers accurately stage the disease and develop an appropriate treatment plan. Here’s a brief explanation of each of the mentioned diagnostic modalities:
- Breast Biopsy: A biopsy is a crucial step in the diagnosis of breast cancer. It involves obtaining a tissue sample from the breast tumor to examine it under a microscope and determine if cancer is present and its specific characteristics.
- Anatomy Pathology: Pathological examination of the biopsy sample allows pathologists to assess the tumor’s type, grade, and other characteristics, providing essential information for staging and treatment decisions.
- Hormonal and Molecular Profile: Determining the hormone receptor status (ER, PR) and HER2 status helps classify the breast cancer subtype and guides targeted treatment options. In the case of TNBC, the absence of hormone receptors and HER2 expression is confirmed.
- Bone Skelelogram: Bone scintigraphy (skelelogram) is used to assess if breast cancer has spread to the bones. A radioactive tracer is injected, and images are taken to detect areas of increased bone activity, indicating possible bone metastases.
- Whole Body Computed Tomography (CT) and PET/CT: These imaging studies are performed to detect metastases in other organs and regions of the body. CT scans provide detailed cross-sectional images, while PET/CT combines CT with positron emission tomography (PET) to highlight areas of increased metabolic activity, such as cancerous lesions.(12- 14)
By combining the information obtained from these diagnostic tests, oncologists can accurately stage TNBC, which helps determine the best treatment approach. Staging takes into account the size and extent of the primary tumor, lymph node involvement, and the presence of distant metastases. Proper staging is crucial in selecting appropriate treatments and predicting the prognosis for patients with TNBC.
Treatment
Patients with TNBC without metastasis and with a negative status axilar (N-)
Treatment for patients with triple-negative breast cancer (TNBC) without metastasis and a negative axillary lymph node status (N-) typically involves a combination of local and systemic therapies. The treatment plan is based on the stage of the tumor, grade, patient’s age, overall health, and individual factors. Here are the general treatment approaches:
Surgery:The primary treatment for localized TNBC is surgery. The goal is to remove the tumor along with a margin of surrounding healthy tissue. Depending on the tumor’s size and location, options may include:
- Lumpectomy: Removal of the tumor and a small portion of surrounding tissue.
- Mastectomy: Removal of the entire breast.
- Sentinel Lymph Node Biopsy: If the axillary lymph nodes are negative on imaging, a sentinel lymph node biopsy may be done to determine if cancer has spread to the nearby lymph nodes. If the sentinel nodes are negative, further lymph node dissection may not be required.
Radiation Therapy: After surgery, radiation therapy may be recommended to target any remaining cancer cells and reduce the risk of local recurrence. This is often used following a lumpectomy.
Systemic Therapy: Since TNBC lacks hormone receptors and HER2 expression, hormonal therapies and HER2-targeted treatments are not effective. Systemic therapy for early-stage TNBC includes chemotherapy, which is typically given after surgery to reduce the risk of cancer recurrence.
Clinical Trials: For some patients with high-risk or more aggressive TNBC, participation in clinical trials evaluating novel therapies may be considered [15, 16].
Radiotherapy
The choice of radiotherapy technique for patients with triple-negative breast cancer (TNBC) depends on various factors, including the size and location of the tumor, the patient’s anatomy, and the specific treatment goals. The common radiotherapy techniques used for breast cancer, including TNBC, are as follows:
- 3D Conformal Radiotherapy (3D-CRT): 3D-CRT uses computerized treatment planning to deliver radiation beams from multiple directions. It shapes the radiation beams to conform closely to the shape of the tumor, minimizing exposure to surrounding healthy tissues.
- Intensity-Modulated Radiation Therapy (IMRT): IMRT is an advanced form of 3D-CRT that allows for even more precise radiation dose shaping. It uses computer-controlled linear accelerators to adjust the intensity of the radiation beams during treatment, delivering varying doses to different areas as needed.
- Proton Therapy: Proton therapy is a specialized form of radiation therapy that uses protons instead of X-rays. Protons have unique physical properties that allow for precise targeting of the tumor while sparing nearby healthy tissues. Proton therapy may be considered in certain cases, especially for patients with complex tumor shapes or those at higher risk of side effects.
The choice between these techniques will be determined by the radiation oncologist and the radiation therapy team based on the individual patient’s needs, tumor characteristics, and the availability of specific technologies at the treatment facility. All of these techniques aim to deliver effective radiation treatment while minimizing the impact on healthy surrounding tissues and organs.
Medical treatments
Treatment protocols for early-stage triple-negative breast cancer (TNBC) may vary depending on the stage of the cancer and the specific characteristics of the tumor. Neoadjuvant and adjuvant chemotherapy are the standard approaches used to treat early TNBC. Here are some common drugs used in these treatment protocols:
1.Neoadjuvant Chemotherapy:
- Anthracycline-based regimens: Drugs like doxorubicin and epirubicin are commonly used in combination with other chemotherapy agents.
- Taxane-based regimens: Drugs like paclitaxel and docetaxel are frequently used in combination with anthracyclines or as single agents.
- Platinum-based regimens: Drugs like cisplatin or carboplatin may be used, particularly in patients with BRCA1 mutations.
- Neoadjuvant chemotherapy is given before surgery to shrink the tumor and increase the likelihood of successful breast-conserving surgery (lumpectomy) while reducing the risk of recurrence.
2.Adjuvant Chemotherapy:
- Anthracycline and/or taxane-based regimens: Similar to neoadjuvant therapy, these drugs are used in the adjuvant setting to reduce the risk of cancer recurrence after surgery.
- Cyclophosphamide and methotrexate: These drugs may be used in specific adjuvant chemotherapy regimens.
The choice of specific drugs and regimens will depend on factors such as the patient’s overall health, age, tumor characteristics, and the potential for side effects. The treatment plan is individualized based on these factors and the oncologist’s clinical judgment.
It’s important to note that ongoing research and clinical trials are continuously evaluating new chemotherapy agents and combinations to improve treatment outcomes for early TNBC. Patients with TNBC are encouraged to discuss the available treatment options, potential side effects, and the benefits of participating in clinical trials with their oncologist. As treatments evolve, more targeted and personalized therapies may become available for TNBC patients [17,18]
[Table I]
| National Comprehensive Cancer Network NCCN Guidelines versión 4.2023 Invasive Breast Cancer |
||
| Systemic Chemotherapy for HR-Positive or -Negative and HER2-Negativesastu | ||
| Preferred Regimens | Other Recommended Regimens | Useful in Certain Circumstances |
Taxanes
Antimetabloites
Microtubule Inhibitors
|
Other Recommended Regimens
|
· Useful in Certain Circumstances
|
Advance Disease
In advanced or metastatic triple-negative breast cancer (TNBC), the treatment approach often involves systemic therapies to target the cancer cells throughout the body. Since TNBC lacks the hormone receptors (ER, PR) and HER2 expression, hormonal therapies and HER2-targeted treatments are not effective. The mainstay of treatment for advanced TNBC includes:
Chemotherapy:
– Taxanes: Drugs like paclitaxel, docetaxel, and nab-paclitaxel are commonly used in the first-line or subsequent lines of treatment for advanced TNBC.
– Gemcitabine: It may be used as a single agent or in combination with other drugs.
– Platinum-based agents: Cisplatin and carboplatin may be used in specific cases, especially in patients with BRCA1 mutations.
– Anthracyclines: In some cases, anthracyclines like doxorubicin or epirubicin may be used in combination regimens.
Immunotherapy
– Immune checkpoint inhibitors: PD-1 inhibitors, such as pembrolizumab, and PD-L1 inhibitors, such as atezolizumab, have shown promising results in some patients with advanced TNBC.
Immunotherapy has emerged as a promising treatment option for triple-negative breast cancer (TNBC), particularly in the advanced or metastatic setting. Immunotherapy works by stimulating the patient’s immune system to recognize and attack cancer cells more effectively. The main type of immunotherapy used in TNBC is immune checkpoint inhibitors.
1. Mechanism of Action:
Immune checkpoint inhibitors block certain proteins on cancer cells or immune cells, such as PD-1 (programmed cell death protein 1) and PD-L1 (programmed death-ligand 1), to prevent cancer cells from evading the immune response. By inhibiting these checkpoints, the immune system can better target and attack cancer cells.
2. Use in Advanced TNBC:
Immunotherapy with checkpoint inhibitors, such as pembrolizumab and atezolizumab, has shown promising results in a subset of patients with advanced TNBC. It is typically used in combination with chemotherapy in patients whose tumors have PD-L1 expression or as a single agent for those with high tumor-infiltrating lymphocytes (TILs).
3. When is Immunotherapy used?
Immunotherapy is generally considered for patients with advanced TNBC who have received prior chemotherapy or for whom chemotherapy is not effective. It may be used as a first-line treatment in combination with chemotherapy or as a subsequent-line treatment when the disease progresses after initial therapy.
4. Where is Immunotherapy Administered?
Immunotherapy is typically administered in a hospital or clinic setting by trained healthcare professionals. It is usually given as an intravenous (IV) infusion over a period of time.
5. Clinical Trials:
Ongoing clinical trials are evaluating different combinations of immunotherapy, targeted therapies, and chemotherapy for TNBC. Clinical trials offer an opportunity for patients to access innovative treatments that may not be widely available yet.
It’s important to note that while immunotherapy has shown promising results for some patients with advanced TNBC, it may not be effective for everyone. The response to immunotherapy can vary based on the individual patient’s tumor characteristics and immune response [19] [Table 2 and 3].
Table 2
| National Comprehensive Cancer Network* NCCN Guidelines Version 4.2023 Invasive Breast Cancer |
||
| SYSTEMIC THERAPY REGIMENS FOR RECURRENT UNRESECTAB LE (LOCAL OR REGIONAL} OR STAGE IV {M1) DISEASEa | ||
| HR-Negative and HER2-Negative {Triple-Negative Breast Cancer; TNBC) | ||
| Setting First Line |
Subtype/Biomarker | Regimen |
| PD-L1 CPS z10g regardless of germ line BRCAmutation statusb |
Pembrolizumab + chemotherapy (albumin-bound paclitaxel, paclltaxel, or gemcifabine and carbopIatin)h (Category 1, preferred) | |
| PD-L1 CPS <10g and no germline BRCA1/2 mutation b | Systemic chemotherapy see BINV-Q (5) | |
| PD-L1 CPS <10 g and germline BRCA1/2 mutationb | PARPi (olaparib, lalazoparib) (Category 1, preferred) Platinum (cisplatln or carboplatin) (Category 1, preferred) |
|
| Second Line | Germline BRCA1/2 mutationb | PARPi (olaparib, lalazoparib) (Category 1, preferred) |
| Any | Saclfuzumab govifecan i (Category 1, preferred) | |
| Systemic chemotherapy see BINV-Q (5) | ||
| No germline BRCA1/2 mutationb and HER2 IHC 1+ or 2+/ISH negatived |
Fam-trastuzumab deruxtecan-nxkie (Category 1, preferred) | |
| Third Line and beyon d | Biomarker positive (ie, MSI-H, NTRK, RET, TMB-H) | Targeted agents see BINV-Q (6) |
| Any | Systemic chemotherapy see BINV-Q (5) | |
|
National Comprehensive
Cancer Network*NCCN Guidelines Version 4.2023 Invasive Breast Cancer |
|||
| DOSING :SYSTEMIC TERAPHY REGIMEN FOR RECURRENT UNRESECTABLE (LOCAL OR REGIONAL) OR STAGE IV (M1) DISEASE HER2-NEGATIVE REGIMEN: |
|||
| Anthracyclines: | Docetaxel 14,15 | AC 24 | Carboplatin/albumin-bound paclitaxel |
| > Doxorubicin 60-70 mg/m2 iv day; cycled every 21 days | > 60—100 mg/m’ IV day 1 > cycled every 21 days |
Doxorubicin 60-70 mg/m2 IV day Cyclophosphide 600mg/m2 IV day 1 cycled every 21 days |
Carboplatin AUC IV on days 1 and 8 Albumin bound paclitaxel 125 mgm2 IV days 1 and 8 Cycled every 21 days |
| > Doxorubicin 20 mg/m2 IV day 1 weekly2 | Docetaxel 16 35 mg/m2 IV day IV weekly for 6 weeks Followed by a 2 week rest then repeat |
EC 25 Epirubcin 75 mg/m2 IV day 1 cyclophosphamide 600 mg/m2 IV day 1 Cycled every 21 days |
Carboplatin/paclitaxel32 ,32 > Paclitaxel 175-200mg/m2 IV day 1 > CorbaplatinAUC 6 IV day 1 > Cycled every 21 days > Paclitaxel 100 mg/m2 IV days 1,8 and 15 > Cycled every 28 days |
| > Liposomal Doxorrubcin3 50 mg/m 2 iv day 1;cycled every 28 days | Albumin-bound paclitaxel 17 18 100 mg/m2 or 125mg/m2 IV days 1,8,15 cycled every 28 days |
Cmf26 Cyclophosphamide 100 mg/2 po days 1-14 Methotrexate 40 mg/m2 IV days 1 and 8 5-fluorouracil 600 mg/m2 IV days 1 and 8 |
See Dosing for additional targeted therapies on BINV-Q (13) |
| Taxanes: | Albumin-bound paclitaxel 17 260 mg/m2 IV cycled every 21 days |
Docetaxel/capecitabin27 Docetaxel 75 mg/m2 IV day 1 Capecitabin 950 mg/m2 po twice daily1-14 Cycled every 21 days |
|
| > Paclitaxel175mg/m2 IV day 1;cycled every 21 days4 | Epirubicin19 60-90mg/m2 IV day 1 cycled every 21 days |
GT28 Paclitaxel 175 mg/m2 IV DAY 1 Gemcitabin 1250 mg/m2 IV day 1 and (following paclitaxel on day 1) Cycled every 21 days |
|
| > Paclitaxel 80mg/m2 IV day I weekly5 | Lxabepilone20 40mg/m2 IV day I cycled every 21 days |
Gemcitabine/carboplatin29 Gemcitabine 1000 mg/2 on day 1 and 8 Carboplatin AUC IV days 1 and 8 Cycled every 21 days |
|
| Antimetabloites: | Sacituzumab govitecan-hziy For TNBC OR HR+/HER2-)21,22 10mg/kg IV on days 1 and 8 cycled every 21 days |
||
| > Capeci tabine61000—1250 mg/m2 PO twice daily days 1—14, cycled every 21 days |
Fam-trastuzumab deruxtecan-nxki For HER2 IHC1+0R 2+/ISH negative)23 cycled every 21 days |
||
| Gemcitabine7 800—1200 mg/m2 IV days 1, 8,and 15; cycled every 28 days | |||
| Microtubule Inhibitors | |||
| > Vinorelbine 8,9 | |||
| > 25 mg/m2 IV day 1 weekly;or > 20-35 mg/m2 IV days 1 and 8; cycled every 21 days;or > 25—30 mg/m2 IV days 1. 8, and 15: cycled ever 28 days > Eribulin10 1.4 mglm2 IV days 1 and 8: cycled ever 21 days Platinum (for TNBC and germline BECA1/2 mutation) > CarbopJatin11 AUC 6 IV on day 1 Cycled every 21-28 days > CarbopJatin12 75mg/m2 IV on day 1 Cycled every 21 • Cyclophosphamide 13 > 50 mg PO daily on days 1—21 > Cycled every 28 days |
|||
| The Selection dosing and administration of anti-cancer agents and the management of associated toxicities are complex. Modification of drug dose and schedule and initiation of supportive care interventions are often necessary because of expected toxicities and individual patient variability, prior treatment and comorbidity. The optimal delivery of anti-cancer agents and the management of associated toxicities in patients with cancer. | |||
Immune Microenvironment
The immune microenvironment in triple-negative breast cancer (TNBC) refers to the complex interactions between the tumor cells and various components of the immune system within the tumor microenvironment. TNBC is characterized by its lack of estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2) expression, making it challenging to target with hormone therapy or HER2-targeted treatments. As a result, the immune system’s role becomes particularly important in shaping the tumor’s behavior and response to treatment.
Key components of the immune microenvironment in TNBC include:
1. Tumor-Infiltrating Lymphocytes (TILs): TILs are immune cells, primarily T cells, that migrate into the tumor and play a crucial role in the antitumor immune response. Higher levels of TILs have been associated with better prognosis and increased responsiveness to immunotherapy in TNBC.
2. Immune Checkpoints: Immune checkpoints are molecules on immune cells that regulate the immune response. Tumor cells often exploit these checkpoints to evade immune detection. Immune checkpoint inhibitors, such as PD-1 and PD-L1 inhibitors, are designed to block these interactions and enhance the immune response against the tumor.(20’
3. Cytokines and Chemokines: These signaling molecules play a role in immune cell communication and recruitment to the tumor site. They can influence the tumor microenvironment and determine whether the immune response is pro- or anti-tumor.
4. Myeloid-Derived Suppressor Cells (MDSCs): MDSCs are a type of immune cell that can suppress the activity of T cells and other immune responses. Their presence in the tumor microenvironment can dampen antitumor immunity.
5. Tumor-Associated Macrophages (TAMs): TAMs are immune cells that are often found in the tumor microenvironment. Depending on their phenotype, they can have either pro-tumor or antitumor effects.
Understanding the immune microenvironment in TNBC is essential for developing targeted therapies and immunotherapies that can harness the patient’s immune system to fight the cancer. Immunotherapy, particularly immune checkpoint inhibitors, has shown promising results in a subset of TNBC patients, especially those with high levels of TILs and PD-L1 expression.
Ongoing research is focused on identifying predictive biomarkers and understanding the complexities of the immune microenvironment in TNBC to optimize treatment approaches and improve patient outcomes. Additionally, combination therapies that target both the tumor cells and the immune microenvironment are being explored as potential strategies to enhance the effectiveness of immunotherapy in TNBC.
3. Targeted Therapies (in clinical trials):
– Poly(ADP-ribose) polymerase (PARP) inhibitors: Drugs like olaparib and talazoparib are being studied in patients with TNBC and BRCA mutations.
– Androgen receptor (AR) inhibitors: Enzalutamide and other AR inhibitors are being investigated for AR-positive TNBC.
It’s important to note that treatment decisions for advanced TNBC are based on factors such as the patient’s overall health, previous treatments, and the extent of disease. Treatment plans are often adjusted based on the response to therapy and the occurrence of side effects.
Additionally, ongoing clinical trials continue to explore new treatment approaches and novel combinations to improve outcomes for patients with advanced TNBC. Patients with advanced TNBC are encouraged to discuss all available treatment options, potential side effects, and the possibility of participating in clinical trials with their healthcare team to make informed decisions about their care.
Targeted therapies
The role of targeted therapies in triple-negative breast cancer (TNBC) is to specifically target certain molecular abnormalities or pathways that contribute to the growth and survival of cancer cells. Unlike chemotherapy, which affects rapidly dividing cells (both cancerous and normal cells), targeted therapies aim to selectively attack cancer cells while sparing healthy cells, potentially leading to more effective and less toxic treatments. However, it’s important to note that targeted therapies are not yet available for all TNBC cases, as research in this field is ongoing. Here are some of the targeted therapies being explored for TNBC.
[Table 4].
|
National Comprehensive Cancer Network* NCCN Guidelines Version 4.2023 Invasive Breast Cancer |
|||||
| ADDITIONAL TARGETED THERAPIES AND ASSOCIATED BIOMARKER TESTING FOR RECURRENT UNRESECTABLE (LOCAL OR REGIONAL) OR STAGE IV (MI) DI SEASE | |||||
| Biomarkers Asson rated with FDA-Appr oved Therapies | |||||
| Breast Cancer Subtype |
Biomarker | Detection | FDA-Approved Agents | NCCN Category of Evidence | NCCN Category of Preference |
| HR-positive/HER2-negativev | PIK3CA activating mutation | PCR (blood or tissue block if blood negative | Alpel +s+ b + In tvesIrant’w‘ | Category 1 | Preferred second or subsequent line therapy |
| HR-positive/HER2-negativex | ESR1 muta lion | NGS, PCR blood) | Eiacestrant
|
Category 2 A | Other recommended regimen |
| Any | NTRK Fusion | FISH, NGS, PCR,(tissue block) | Larotrectiniby
Entrectinib+ |
Category 2A | Useful in certain circumstances |
| Any | MSI -H/dMMR
|
INC, NGS, PCR (tissue block) | Dostarlimab-gxly bb | Category 2A | |
| Any | TMB-H (z10 mut/mb)
|
NGS | Pembroiizumabzaa | Category 2A | |
| Any | RET-fusion
|
NGS | Selpercatinibcc
|
Category 2A | |
- PARP Inhibitors: Poly(ADP-ribose) polymerase (PARP) inhibitors, such as olaparib and talazoparib, are being studied in patients with TNBC and BRCA mutations. PARP inhibitors block an enzyme that helps repair damaged DNA, leading to cancer cell death. These drugs may be effective in patients with BRCA1/2 mutations, which are found in a subset of TNBC cases.
- Androgen Receptor (AR) Inhibitors: Some TNBC tumors express the androgen receptor (AR). AR inhibitors, like enzalutamide, are being investigated as potential targeted therapies for AR-positive TNBC.
- EGFR Inhibitors: Epidermal growth factor receptor (EGFR) inhibitors have been studied in TNBC due to EGFR overexpression in some tumors. However, results have been mixed, and more research is needed to determine their efficacy.
- Angiogenesis Inhibitors: Bevacizumab is an example of an angiogenesis inhibitor, which targets the process of forming new blood vessels that feed cancer tumors. It has been studied in combination with chemotherapy for TNBC.
- Immunotherapy: As mentioned earlier, immune checkpoint inhibitors like pembrolizumab and atezolizumab are a form of targeted therapy that modulates the immune system’s response to cancer cells. They have shown promise in a subset of patients with advanced TNBC [21]
Oligometastatic Disease
The treatment of triple-negative breast cancer (TNBC) in patients with oligometastatic disease, meaning a limited number of metastases in specific sites like the nervous system or bones, is a complex and individualized process. The management approach depends on various factors, including the extent and location of metastases, the patient’s overall health, and treatment goals. Here are some treatment strategies commonly used for oligometastatic TNBC:
1. Surgery: In some cases, surgical resection of isolated metastatic lesions may be considered. This can be applicable to brain metastases or certain bone metastases where surgery can help alleviate symptoms and improve outcomes.
2. Radiation Therapy: Localized radiation therapy is often used to treat specific metastatic sites, such as brain metastases or painful bone lesions. Techniques like stereotactic radiosurgery (SRS) or stereotactic body radiation therapy (SBRT) can deliver precise, high-dose radiation to the targeted areas while sparing surrounding healthy tissue.
3. Systemic Therapy: Depending on the extent of metastases and the response to initial treatments, systemic therapies such as chemotherapy or immunotherapy may be used to control both the primary tumor and metastases. Clinical trials evaluating targeted therapies may also be considered for specific subgroups.
4. Immunotherapy: Immune checkpoint inhibitors, like pembrolizumab or atezolizumab, have shown efficacy in some patients with advanced TNBC, including those with brain metastases.
5. Targeted Therapies: For patients with specific molecular features, such as BRCA mutations, PARP inhibitors like olaparib or talazoparib may be considered.
6. Clinical Trials: Participation in clinical trials can offer access to innovative treatments and potentially improve outcomes for patients with oligometastatic TNBC.
Novel therapies
Researchers and clinicians have been actively exploring novel therapeutic approaches for triple-negative breast cancer (TNBC) to improve treatment outcomes. Some of the promising novel approaches include:
Immunotherapy Combinations: Studies are investigating combinations of immune checkpoint inhibitors with other immunotherapies, chemotherapy, or targeted therapies to enhance the immune response against TNBC.
Antibody-Drug Conjugates (ADCs): ADCs are a type of targeted therapy that combines an antibody that recognizes specific proteins on cancer cells with a potent chemotherapy drug. ADCs are being developed to selectively deliver chemotherapy directly to TNBC cells while minimizing toxicity to healthy tissues.
Bispecific Antibodies: These antibodies are engineered to bind to two different targets simultaneously. Bispecific antibodies are being explored to enhance the immune response and target multiple pathways involved in TNBC growth and survival.
Tumor-Infiltrating Lymphocyte (TIL) Therapy: TIL therapy involves isolating and expanding TILs from a patient’s tumor, then infusing them back into the patient to enhance the immune response against cancer cells.
Adoptive T-cell Therapy: This approach involves engineering a patient’s T cells in the laboratory to express specific receptors that target TNBC cells, increasing their ability to attack the cancer.
Epigenetic Therapies: Epigenetic modifications play a role in gene expression changes in cancer. Drugs that target epigenetic changes are being investigated as potential therapies for TNBC.
Antibody-Drug Radioimmunotherapy (ADR): ADR combines targeted antibodies with radioactive isotopes to deliver radiation directly to cancer cells, limiting radiation exposure to healthy tissues.
Combination Therapies: Researchers are studying various combinations of existing drugs, such as chemotherapy, targeted therapies, and immunotherapies, to optimize treatment responses in TNBC. [Table 5].
|
National Comprehensive |
|||||
| EMERGING BIOMARKERS TO IDENTIFY NOVEL THERAPIES FOR PATIENTS WITH STAGEV1(M1) DISEASE | |||||
| Breast Cancer Subtype | Emerging Biomarkers | NGSee | Potential targeted therapy dd | NCCN Category of Evidence | NCCN Category of Preference |
|
ER+/HER2- ER-/HER2- |
HER2 activating mutations | NGSee |
Neratinib± fulvestrantff Neratinib±trastuzumabtgg |
Category 2B |
Useful in certain circumstances · If ER+HER2-,in patients who have already received CDK4/6 inhibitor theraphy |
| Any | Somatic BRCA1/2 mutations | NGSee | olaparibhh | Category 2B | Useful in certain circumstances |
| Any | Germline PALB2 | Germline Sequencing | olaparibhh | Category 2B | Useful in certain circumstances |
It’s important to note that clinical trials play a significant role in evaluating the safety and efficacy of these novel therapeutic approaches. Participating in clinical trials can provide eligible patients with access to promising treatments that may not be available through standard care [22].
The cost of breast cancer treatment
The cost of breast cancer treatment, including for triple-negative breast cancer (TNBC), can vary significantly depending on various factors such as the stage of cancer, the specific treatments used, the country or region where treatment is received, the type of healthcare insurance, and individual medical circumstances.
The cost of treatment can include expenses related to:
1.Surgery: Costs associated with mastectomy or lumpectomy, hospitalization, anesthesia, and follow-up care.
2.Chemotherapy: Costs for chemotherapy drugs, infusion or administration fees, and supportive medications.
3.Radiation Therapy: Costs for radiation treatments and related services.
4.Targeted Therapies and Immunotherapies: Costs for targeted drugs or immunotherapies used in the treatment.
5.Imaging and Diagnostic Tests: Costs for mammograms, biopsies, PET/CT scans, and other tests used for diagnosis and monitoring.
6.Supportive Care: Costs for managing side effects, pain management, and other supportive services.
7.Hospitalization and Inpatient Care: Costs for hospital stays, surgeries, and related services.
8.Doctor Visits and Consultations: Costs for oncologist visits, surgeon consultations, and other medical appointments.
The total cost of TNBC treatment can range from tens of thousands to hundreds of thousands of dollars, depending on the factors mentioned above. It’s essential for patients to work closely with their healthcare providers and insurance companies to understand their treatment costs and coverage.
Additionally, some countries have national health systems or assistance programs that may provide financial support or subsidized treatment options for cancer patients. Patient advocacy groups and nonprofit organizations may also offer resources and support to help manage the financial burden of cancer treatment.
It’s important to remember that the cost of treatment should never be a barrier to seeking necessary medical care. If you or someone you know is facing financial challenges related to TNBC treatment, consider reaching out to social workers or patient support organizations to explore available resources and assistance programs [23].
Conclusion
In conclusion, triple-negative breast cancer (TNBC) is a subtype of breast cancer that lacks expression of hormone receptors (ER, PR) and human epidermal growth factor receptor 2 (HER2). TNBC is characterized by its aggressive behavior and limited treatment options, making it a challenging form of breast cancer to manage.
Diagnosing TNBC involves a combination of imaging studies, biopsy, and pathological examination. Treatment for early-stage TNBC typically includes surgery, radiation therapy, and chemotherapy. In advanced TNBC, systemic therapies like chemotherapy and immunotherapy, particularly immune checkpoint inhibitors, are often used.
Targeted therapies, such as PARP inhibitors and AR inhibitors, are being studied in clinical trials for specific subsets of TNBC cases, providing hope for more tailored and effective treatments in the future.
The prognosis for patients with TNBC varies based on the stage of the disease and individual patient characteristics. Early detection and timely treatment are crucial for improving outcomes.
Ongoing research and clinical trials continue to advance our understanding of TNBC’s biology and identify new treatment strategies, giving hope for better outcomes and quality of life for patients with this aggressive breast cancer subtype [24].
Patients with TNBC should work closely with a multidisciplinary healthcare team to develop individualized treatment plans, taking into account the latest advancements and available treatment options. Support from patient advocacy groups and nonprofit organizations can also be valuable in navigating the challenges associated with TNBC and its treatment.
References
- Dent R, Trudeau M, Pritchard KI, Sun P, Hanna et al. (2007) Triple-negative breast cancer: clinical features and patterns of recurrence. Clin Cancer Res 13: 4429-4434.
- Bauer KR, Brown M, Cress RD, Parise CA, Caggiano V (2007) Descriptive analysis of estrogen receptor (ER)-negative, progesterone receptor (PR)-negative, and HER2-negative invasive breast cancer, the so-called triple-negative phenotype. Cancer 109:1721-1728.
- Lehmann BD, Bauer JA, Chen X (2011) Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J Clin Invest 121: 2750-2767.
- Foulkes WD, Smith IE, Reis-Filho JS (2010) Triple-negative breast cancer. N Engl J Med 363: 1938-1948.
- Carey LA, Perou CM, Livasy CA, Robert C, Conway et al. (2006) Race, breast cancer subtypes, and survival in the Carolina Breast Cancer Study. JAMA 295: 2492-2502.
- Dent R, Hanna WM, Trudeau M (2009) Pattern of metastatic spread in triple-negative breast cancer. Breast Cancer Res Treat 115: 423-428.
- Liedtke C, Mazouni C, Hess KR, Lajos P, Massimo S et al. (2008) Response to neoadjuvant therapy and long-term survival in patients with triple-negative breast cancer. J Clin Oncol 26:1275-1281.
- Ovcaricek T, Frkovic SG, Matos E, Mozina B, Borstnar S (2011) Triple negative breast cancer–prognostic factors and survival. Radiol Oncol 45: 46-52.
- Rakha EA, El-Sayed ME, Green AR, Lee AH, Robertson JF, Ellis IO (2007) Prognostic markers in triple-negative breast cancer. Cancer 109: 25-32.
- Viale G, Rotmensz N, Maisonneuve P, Marco C, Aron G et al. (2009) Invasive ductal carcinoma of the breast with the “triple-negative” phenotype: prognostic implications of EGFR immunoreactivity. Breast Cancer Res Treat 116: 317-328.
- Isakoff SJ, Mayer EL, He L, Leif W Else, Paul Goss, et al. (2015) TBCRC009: A Multicenter Phase II Clinical Trial of Platinum Monotherapy With Biomarker Assessment in Metastatic Triple-Negative Breast Cancer. J Clin Oncol. 33:1902-1909.
- Anders CK, Carey LA. Biology, metastatic patterns, and treatment of patients with triple-negative breast cancer. Clin Breast Cancer. 2009;9 Suppl 2:S73-81.
- Dent R, Trudeau M, Pritchard KI, Ping S, Steven N, et al. (2007) Triple-negative breast cancer: clinical features and patterns of recurrence. Clin Cancer Res 13: 4429-4434.
- Lehmann BD, Bauer JA, Chen X, Jennifer A, Shyr Yu, et al. (2011) Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J Clin Invest 121: 2750-2767.
- Pal SK, Childs BH, Pegram M (2011) Triple negative breast cancer: unmet medical needs. Breast Cancer Res Treat. 125: 627-636.
- Cortes J, Fumoleau P, Bianchi GV, Petrella TM, Gelmon K (2019) The role of nab-paclitaxel in the treatment of triple-negative breast cancer: Harnessing the albumin-bound protein’s unique pharmacological and tumor-targeting attributes to improve patient outcomes. Breast Cancer Res21:123.
- Bauer KR, Brown M, Cress RD, Parise CA, Caggiano V (2007) Descriptive analysis of estrogen receptor (ER)-negative, progesterone receptor (PR)-negative, and HER2-negative invasive breast cancer, the so-called triple-negative phenotype. Cancer 109: 1721-1728.
- Carey LA, Perou CM, Livasy CA, Robert K, David et al. (2006) Race, breast cancer subtypes, and survival in the Carolina Breast Cancer Study. JAMA 295: 2492-2502.
- Lehmann BD, Pietenpol JA (2014) Identification and use of biomarkers in treatment strategies for triple-negative breast cancer subtypes. J Pathol 232: 142-150.
- Dent R, Hanna WM, Trudeau M, et al. (2009) Pattern of metastatic spread in triple-negative breast cancer. Breast Cancer Res Treat 115: 423-428.
- Sharma P (2016) Biology and Management of Patients With Triple-Negative Breast Cancer. The Oncologist. 21:1050-1062.
- Tutt A, Tovey H, Cheang MCU, et al. (2018) Carboplatin in BRCA1/2-mutated and triple-negative breast cancer BRCAness subgroups: the TNT Trial. Nat Med 24: 628-637.
- Cortazar P, Zhang L, Untch M, et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet 384:164-172.
- Lehmann BD, Jovanović B, Chen X, et al. (2016) Refinement of Triple-Negative Breast Cancer Molecular Subtypes: Implications for Neoadjuvant Chemotherapy Selection. PLoS ONE :e0157368.
*Corresponding author: Dr. Adrian P. Hunis, School of Medicine, Universidad de Buenos Aires (UBA), Emeritus Member of ASCO, Emeritus Member of ESMO. E-mail:aphunis@gmail.com
Citation: Dr Adrian P. Hunis (2023) Triple-Negative Breast Cancer (TNBC) Arc Can Res Med 4: 012. DOI: https://doi.org/10.58735/acrmr112
Received: July 19, 2023; Accepted: Aug 26, 2023, Published: Sep 02, 2023
Copyright: © 2023 Dr Adrian P. Hunis. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits un-restricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Adrian P. Hunis1*, Melisa Hunis2
1School of Medicine, Universidad de Buenos Aires (UBA), Emeritus Member of ASCO, Emeritus Member of ESMO
2 Universidad Maimonides (UMAI)